当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
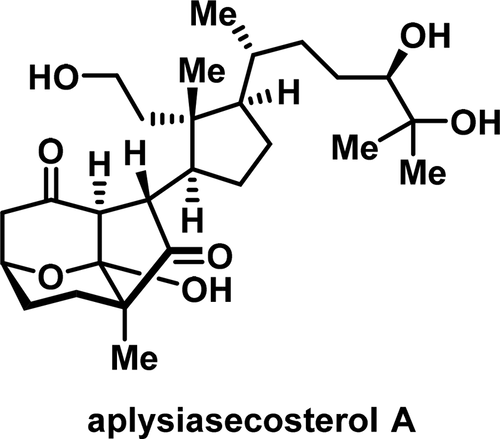
Total Synthesis of Aplysiasecosterol A
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-25 , DOI: 10.1021/jacs.8b05070 Zhaohong Lu 1 , Xiang Zhang 1 , Zhicong Guo 1 , Yu Chen 1 , Tong Mu 1 , Ang Li 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-06-25 , DOI: 10.1021/jacs.8b05070 Zhaohong Lu 1 , Xiang Zhang 1 , Zhicong Guo 1 , Yu Chen 1 , Tong Mu 1 , Ang Li 1
Affiliation
|
Aplysiasecosterol A (1) is a structurally unusual 9,11-secosteroid isolated from the sea hare Aplysia kurodai. We have accomplished the first and asymmetric total synthesis of 1 in a convergent fashion. The left-hand segment bearing three adjacent stereocenters was constructed through desymmetrizing reduction, ketalization, and radical cyclization. A strategy of asymmetric 2-bromoallylation followed by spontaneous desymmetrizing lactolization enabled a more expeditious access to this segment. The right-hand segment was prepared through two different approaches: one featuring Myers alkylation and Suzuki-Miyaura coupling and the other relying upon Aggarwal lithiation-borylation and Zweifel-Evans olefination. The two fragments were coupled by a Reformatsky type reaction. The three consecutive stereocenters embedded in the central domain of 1 were generated by an iron-mediated, hydrogen atom transfer based radical cyclization reaction.
中文翻译:

海兔甾醇 A 的全合成
Aplysiasecosterol A (1) 是一种从海兔 Aplysia kurodai 中分离出来的结构不寻常的 9,11-secosteroid。我们以收敛的方式完成了 1 的第一次非对称全合成。带有三个相邻立体中心的左侧部分是通过去对称还原、缩酮化和自由基环化构建的。不对称 2-溴烯丙基化后自发去对称化乳糖醇化的策略使得可以更快速地进入该部分。右侧部分是通过两种不同的方法制备的:一种采用 Myers 烷基化和 Suzuki-Miyaura 偶联,另一种依靠 Aggarwal 锂化-硼酸化和 Zweifel-Evans 烯化。两个片段通过 Reformatsky 型反应偶联。
更新日期:2018-06-25
中文翻译:

海兔甾醇 A 的全合成
Aplysiasecosterol A (1) 是一种从海兔 Aplysia kurodai 中分离出来的结构不寻常的 9,11-secosteroid。我们以收敛的方式完成了 1 的第一次非对称全合成。带有三个相邻立体中心的左侧部分是通过去对称还原、缩酮化和自由基环化构建的。不对称 2-溴烯丙基化后自发去对称化乳糖醇化的策略使得可以更快速地进入该部分。右侧部分是通过两种不同的方法制备的:一种采用 Myers 烷基化和 Suzuki-Miyaura 偶联,另一种依靠 Aggarwal 锂化-硼酸化和 Zweifel-Evans 烯化。两个片段通过 Reformatsky 型反应偶联。



























 京公网安备 11010802027423号
京公网安备 11010802027423号