当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic hydrogen evolution from neutral aqueous solution by a water-soluble cobalt(ii) porphyrin†
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8se00253c Belete B. Beyene 1, 2, 3, 4, 5 , Chen-Hsiung Hung 1, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8se00253c Belete B. Beyene 1, 2, 3, 4, 5 , Chen-Hsiung Hung 1, 2, 3, 4
Affiliation
|
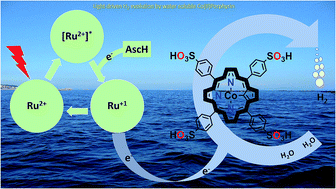
Efficient storage of solar energy via light-driven hydrogen evolution is an attractive and promising strategy to address challenges related to increasing global energy demand. Herein, we report a highly active homogeneous photocatalytic hydrogen evolution system comprising a water-soluble cobalt(II) porphyrin, CoTPPS, as a molecular catalyst, [Ru(bpy)3]2+ as a photosensitizer, and ascorbic acid as a sacrificial electron donor. Under optimized conditions, a hydrogen evolution rate of 1.3 μmol min−1, a TOF of 120.8 min−1, and a TON of 6410 are obtained in 1 M phosphate buffer at pH 6.8 by irradiation with an LED light source at 420 nm and 0.6 W cm−2. The studies on the effects of pH and catalyst concentration showed that optimal H2 evolution efficiency was achieved at a pH of 6.8 and a catalyst concentration of 1.5 μM. Moreover, the kinetics of the fluorescence emission quenching experiment indicates that the rate of reductive quenching of [Ru(bpy)3]2+* by ascorbate (7.5 × 106 s−1) is higher than that of oxidative quenching by CoTPPS (1.7 × 104 s−1). As also supported by redox potential energy level analysis, a mechanistic pathway was proposed in which reduction of photo-excited [Ru(bpy)3]2+* by ascorbic acid produces [Ru(bpy)3]1+, which is a strong reductant and will subsequently reduce CoTPPS to generate cobalt-hydride for hydrogen evolution after protonation.
中文翻译:

从由水溶性钴(中性水溶液的光催化氢析出II)卟啉†
通过光驱制氢有效地存储太阳能是一种有吸引力且有前途的战略,可以应对与全球能源需求增长有关的挑战。在本文中,我们报告了一种高活性的均相光催化制氢系统,该系统包括水溶性钴(II)卟啉,CoTPPS作为分子催化剂,[Ru(bpy)3 ] 2+作为光敏剂以及抗坏血酸作为牺牲电子。捐赠者。在优化的条件下,通过用420 nm和0.6的LED光源照射,在pH值为6.8的1 M磷酸盐缓冲液中,制氢速率为1.3μmolmin -1,TOF为120.8 min -1和TON为6410。宽cm -2。pH和催化剂浓度影响的研究表明,在6.8的pH和1.5μM的催化剂浓度下,可以获得最佳的H 2放出效率。此外,荧光发射猝灭实验的动力学表明,[Ru(bpy)3 ] 2+ *被抗坏血酸盐(7.5×10 6 s -1)的还原猝灭速率高于通过CoTPPS的氧化猝灭(1.7)。 ×10 4 s -1)。还得到氧化还原势能分析的支持,提出了一种机制途径,其中抗坏血酸还原光激发的[Ru(bpy)3 ] 2+ *产生[Ru(bpy)3] 1+,它是一种强还原剂,随后会还原CoTPPS,从而生成质子化后用于氢释放的氢化钴。
更新日期:2018-06-25
中文翻译:

从由水溶性钴(中性水溶液的光催化氢析出II)卟啉†
通过光驱制氢有效地存储太阳能是一种有吸引力且有前途的战略,可以应对与全球能源需求增长有关的挑战。在本文中,我们报告了一种高活性的均相光催化制氢系统,该系统包括水溶性钴(II)卟啉,CoTPPS作为分子催化剂,[Ru(bpy)3 ] 2+作为光敏剂以及抗坏血酸作为牺牲电子。捐赠者。在优化的条件下,通过用420 nm和0.6的LED光源照射,在pH值为6.8的1 M磷酸盐缓冲液中,制氢速率为1.3μmolmin -1,TOF为120.8 min -1和TON为6410。宽cm -2。pH和催化剂浓度影响的研究表明,在6.8的pH和1.5μM的催化剂浓度下,可以获得最佳的H 2放出效率。此外,荧光发射猝灭实验的动力学表明,[Ru(bpy)3 ] 2+ *被抗坏血酸盐(7.5×10 6 s -1)的还原猝灭速率高于通过CoTPPS的氧化猝灭(1.7)。 ×10 4 s -1)。还得到氧化还原势能分析的支持,提出了一种机制途径,其中抗坏血酸还原光激发的[Ru(bpy)3 ] 2+ *产生[Ru(bpy)3] 1+,它是一种强还原剂,随后会还原CoTPPS,从而生成质子化后用于氢释放的氢化钴。











































 京公网安备 11010802027423号
京公网安备 11010802027423号