Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-06-23 , DOI: 10.1016/j.jcat.2018.06.011 Akram Heydari-turkmani , Saeed Zakavi
|
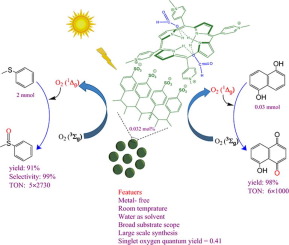
The first solid state porphyrin dication of a weak carboxylic acid was synthesized through the reaction of meso-tetrakis(N-methylpyridinium-4-yl)porphyrin (H2TMPyP) supported on the sodium salt of Amberlyst 15 nanoparticles (nanoAmbSO3Na) with formic acid. The polymer-porphyrin hybrid compound, nanoAmbSO3@H2TMPyP(HCOOH)2, was characterized by FT-IR and diffuse reflectance (DR) UV–vis spectroscopy, EDX, BET, DLS, FESEM and TGA methods. Interestingly, the diacid was quite stable towards decomposition to the corresponding porphyrin and carboxylic acid in neat water as well as in acetonitrile. The protonation reaction was accompanied with the shift of the Soret band from 435 to 454 nm in DR UV–vis spectrum and a rapid color change from red brown to green. A particle size of 190 and <50 nm was estimated for the nanoparticles by DLS and FESEM, respectively. Also, the macroporous structure of the catalyst was revealed by BET experiments. nanoAmbSO3@H2TMPyP(HCOOH)2 was used as photosensitizer for the highly chemoselective and large scale aerobic oxidation of sulfides to the corresponding sulfoxide in water within 3 h at room temperature. A conversion of ca. 95% was observed for the oxidation of sulfides to their corresponding sulfoxide in water with a selectivity of ca. 100%. The use of acetonitrile as solvent led to a significant decrease in the conversion values while retaining selectivity. The higher efficiency of the catalyst in water was in accord with greater singlet oxygen quantum yield (ΦΔ) value of the photosensitizer in this solvent (ΦΔ = 0.41) relative to that in acetonitrile (ΦΔ = 0.08). Apparently, cooperative acid catalysis caused by the protic solvent and porphyrin diacid is involved in the preference of water over acetonitrile as the solvent for efficient oxidation of sulfides to sulfoxides. Furthermore, the photosensitizer was used for the efficient oxidation of 1,5-dihydroxynaphthalene (DHN) in water within 15 min. The photocatalyst was remarkably stable towards oxidative degradation so that it could be recovered and reused for at least 5 times without loss of activity. It is noteworthy that large scale photooxidation of sulfides (TON ≈ 5 × 3000) and DHN (TON ≈ 6 × 1000) were achieved in water, using nanoAmbSO3@H2TMPyP(HCOOH)2 as the photosensitizer.
中文翻译:

第一个固态卟啉-弱酸分子复合物:一种新型的无金属,纳米级且多孔的光催化剂,用于水中的大规模需氧氧化
通过负载在Amberlyst 15纳米颗粒(nanoAmbSO 3 Na)的钠盐上的间四(N-甲基吡啶-4-基)卟啉(H 2 TMPyP )与甲酸。的卟啉聚合物的杂化化合物,nanoAmbSO 3 @H 2 TMPyP(HCOOH)2的特征在于FT-IR和漫反射紫外可见光谱,EDX,BET,DLS,FESEM和TGA方法。有趣的是,二酸在纯净水和乙腈中对分解成相应的卟啉和羧酸非常稳定。质子化反应伴随着DR UV-vis光谱中Soret谱带从435 nm移至454 nm,颜色快速从红棕色变为绿色。通过DLS和FESEM分别估计纳米颗粒的粒径为190nm和<50nm。同样,通过BET实验揭示了催化剂的大孔结构。nanoAmbSO 3 @H 2 TMPyP(HCOOH)2在室温下3小时内,H2O3被用作光敏剂,用于水中的硫化物高度化学选择性和大规模好氧氧化为相应的亚砜。大约是a的转换。观察到95%的硫化物在水中被氧化为相应的亚砜,选择性为。100%。乙腈作为溶剂的使用导致转化率值显着降低,同时保持了选择性。在水中的催化剂的效率更高是具有更大的单线态氧量子产率一致(Φ Δ)在该溶剂中的光敏剂的值(Φ Δ = 0.41)相对于在乙腈(Φ Δ = 0.08)。显然,质子溶剂和卟啉二酸引起的协同酸催化作用涉及水相对于乙腈的偏好,后者是将硫化物有效氧化成亚砜的溶剂。此外,光敏剂用于在15分钟内有效氧化水中的1,5-二羟基萘(DHN)。光催化剂对氧化降解非常稳定,因此可以回收并重复使用至少5次,而不会损失活性。值得注意的是硫化物的规模大的光氧化(TON≈5×3000)和DHN(TON≈6×1000)在水中来实现,使用nanoAmbSO 3 @H 2 TMPyP(HCOOH)2作为光敏剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号