Journal of Catalysis ( IF 6.5 ) Pub Date : 2018-06-26 , DOI: 10.1016/j.jcat.2018.06.009 Daniel T. Bregante , Ami Y. Patel , Alayna M. Johnson , David W. Flaherty
|
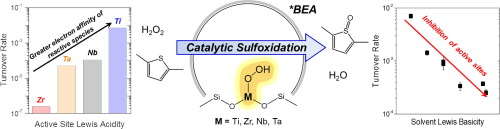
Group 4 (Ti and Zr) and 5 (Nb and Ta) atoms substituted into the *BEA zeolite framework (M-BEA) irreversibly activate hydrogen peroxide (H2O2) and form pools of metal-hydroperoxide (M-OOH) and peroxide (M-(η2-O2)) intermediates active for the oxidation of 2,5-dimethylthiophene (C6H8S), a model reactant representative of organosulfur species in fossil reserves and chemical weapons. Sequential oxidation pathways convert C6H8S into 2,5-dimethylthiophene oxide (C6H8SO) and subsequently into 2,5-dimethylthiophene dioxide by oxidative dearomatization. Oxidation rates measured as functions of reactant concentrations together with in situ UV–vis spectra show that all M-BEA activate H2O2 to form pools of M-OOH and M-(η2-O2), which then react with either C6H8S or H2O2 to form the sulfoxide or to decompose into H2O and O2, respectively. Turnover rates for C6H8S oxidation and H2O2 decomposition both increase exponentially with the electron affinity of the active site, which is quantitatively probed via the adsorption enthalpy for deuterated acetonitrile to active sites. C6H8S oxidation rates depend also on the nucleophilicity of the solvent used, and rates decrease in the order acetonitrile > p-dioxane ∼ acetone > ethanol ∼ methanol. In situ UV–vis spectra show that highly nucleophilic solvent molecules compete effectively for active sites, inhibit H2O2 activation and formation of reactive M-OOH and M-(η2-O2) species, and give lower turnover rates. Consequently, this work shows that turnover rates for sulfoxidation are highest when highly electrophilic active sites (i.e., stronger Lewis acids) are paired with weakly nucleophilic solvents, which can guide the design of increasingly productive catalytic systems for sulfide oxidation.
中文翻译:

用过氧化氢将4和5组骨架取代的沸石催化催化噻吩氧化:金属路易斯酸度和溶剂路易斯碱度影响的机理和光谱学证据
取代入* BEA沸石骨架(M-BEA)的第4组(Ti和Zr)和5(Nb和Ta)原子不可逆地激活过氧化氢(H 2 O 2)并形成金属过氧化氢(M-OOH)和过氧化物(M-(η 2 -O 2))的中间体活性2,5-二甲基噻(C氧化6 ħ 8 S),一个模型反应物代表在化石储量和化学武器的有机硫物质。顺序氧化途径将C 6 H 8 S转化为2,5-二甲基噻吩氧化物(C 6 H 8SO 2),然后通过氧化脱芳烃作用转化为2,5-二甲基噻吩二氧化物。作为反应物浓度的函数原位一起测量的UV-vis光谱显示所有的M-BEA激活ħ氧化速率2 Ò 2至M-OOH和M-的形式池(η 2 -O 2),然后与任一反应C 6 H 8 S或H 2 O 2分别形成亚砜或分解为H 2 O和O 2。C 6 H 8 S氧化和H 2 O 2的周转率分解均随活性位点的电子亲和力呈指数增加,这通过氘化乙腈对活性位点的吸附焓进行定量探测。C 6 H 8 S的氧化速率还取决于所用溶剂的亲核性,并且速率降低的顺序依次为乙腈> 对二恶烷〜丙酮>乙醇〜甲醇。原位的UV-vis光谱显示高度亲核的溶剂分子为有效活性位点竞争,抑制ħ 2 ö 2激活和反应性M-OOH和M-的形成(η 2 -O 2)物种,并提供较低的周转率。因此,这项工作表明,当高度亲电的活性位点(即较强的路易斯酸)与弱亲核溶剂配对时,硫氧化的转化率最高,这可以指导硫化物氧化生产效率越来越高的催化体系的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号