Food Research International ( IF 7.0 ) Pub Date : 2018-06-25 , DOI: 10.1016/j.foodres.2018.06.056 Adrienne L. Voelker , Jenna Miller , Cordelia A. Running , Lynne S. Taylor , Lisa J. Mauer
|
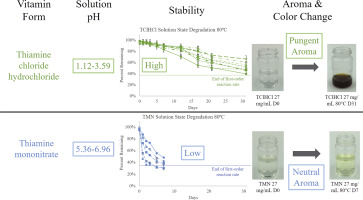
Two types of thiamine (vitamin B1) salts, thiamine mononitrate (TMN) and thiamine chloride hydrochloride (TClHCl), are used to enrich and fortify food products. Both of these thiamine salt forms are sensitive to heat, alkali, oxygen, and radiation, but differences in stability between them have been noted. It was hypothesized that stability differences between the two thiamine salts could be explained by differences in solubility, solution pH, and activation energies for degradation. This study directly compared the stabilities of TMN and TClHCl in solution over time by documenting the impact of concentration and storage temperature on thiamine degradation and calculating reaction kinetics. Solutions were prepared containing five concentrations of each thiamine salt (1, 5, 10, 20, and 27 mg/mL), and three additional concentrations of TClHCl: 100, 300, and 500 mg/mL. Samples were stored at 25, 40, 60, 70, and 80 °C for up to 6 months. Degradation was quantified over time by high-performance liquid chromatography, and percent thiamine remaining was used to calculate reaction kinetics. First-order reaction kinetics were found for both TMN and TClHCl. TMN degraded significantly faster than TClHCl at all concentrations and temperatures. For example, in 27 mg/mL solutions after 5 days at 80 °C, only 32% of TMN remained compared to 94% of TClHCl. Activation energies and solution pHs were 21–25 kcal/mol and pH 5.36–6.96 for TMN and 21–32 kcal/mol and pH 1.12–3.59 for TClHCl. TClHCl degradation products had much greater sensory contributions than TMN degradation products, including intense color change and potent aromas, even with considerably less measured vitamin loss. Different peak patterns were present in HPLC chromatograms between TMN and TClHCl, indicating different degradation pathways and products. The stability of essential vitamins in foods is important, even more so when degradation contributes to sensory changes, and this study provides a direct comparison of the stability of the two thiamine salts used to fortify foods in environments relevant to the processing and shelf-life of many foods.
中文翻译:

溶液中两种硫胺盐(单硝酸硫胺盐和氯化硫胺盐酸盐)的化学稳定性和反应动力学
两种硫胺素(维生素B 1)盐,单硝酸硫胺素(TMN)和氯化硫胺盐酸盐(TClHCl)用于丰富和强化食品。这两种硫胺盐形式均对热,碱,氧气和辐射敏感,但已注意到它们之间的稳定性差异。假设可以通过溶解度,溶液pH值和降解活化能的差异来解释两种硫胺盐之间的稳定性差异。通过记录浓度和储存温度对硫胺素降解的影响并计算反应动力学,本研究直接比较了溶液中TMN和TClHCl随时间的稳定性。制备的溶液含有五种浓度的每种硫胺盐(1、5、10、20和27 mg / mL),以及三种额外的TClHCl浓度:100、300和500 mg / mL。样品分别在25、40、60、70和80°C下保存6个月。通过高效液相色谱法对降解随时间的变化进行定量,并将剩余的硫胺素百分比用于计算反应动力学。发现TMN和TClHCl都具有一级反应动力学。在所有浓度和温度下,TMN的降解速度均明显快于TClHCl。例如,在80°C下放置5天后,在27 mg / mL溶液中,仅残留32%的TMN,而94%的TClHCl。对于TMN,活化能和溶液的pH值分别为21–25 kcal / mol和pH 5.36–6.96,对于TClHCl,其活化能和溶液pH为1.13–3.59。TClHCl降解产物比TMN降解产物具有更大的感官贡献,包括强烈的颜色变化和强烈的香气,即使维生素损失明显减少。TMN和TClHCl之间的HPLC色谱图中存在不同的峰型,表明不同的降解途径和产物。食品中必需维生素的稳定性非常重要,当降解有助于感官变化时,这一点就显得尤为重要。本研究直接比较了用于强化食品的两种硫胺盐在与食品加工和货架期相关的环境中的稳定性。许多食物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号