当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anion Recognition by Organometallic Calixarenes: Analysis from Relativistic DFT Calculations
Organometallics ( IF 2.5 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.organomet.8b00292 Alexandre O. Ortolan 1 , Ina Øestrøm 1 , Giovanni F. Caramori 1 , Renato L. T. Parreira 2 , Alvaro Muñoz-Castro 3 , F. Matthias Bickelhaupt 4, 5
Organometallics ( IF 2.5 ) Pub Date : 2018-06-25 , DOI: 10.1021/acs.organomet.8b00292 Alexandre O. Ortolan 1 , Ina Øestrøm 1 , Giovanni F. Caramori 1 , Renato L. T. Parreira 2 , Alvaro Muñoz-Castro 3 , F. Matthias Bickelhaupt 4, 5
Affiliation
|
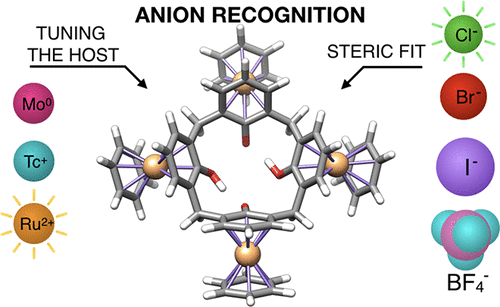
The physical nature of the noncovalent interactions involved in anion recognition was investigated in the context of metalated calix[4]arene hosts, employing Kohn–Sham molecular orbital (KS-MO) theory, in conjunction with a canonical energy decomposition analysis, at the dispersion-corrected DFT level of theory. Computed data evidence that the most stable host–guest bonding occurs in ruthenium complexed hosts, followed by technetium and molybdenum metalated macrocyclic receptors. Furthermore, the guest’s steric fit in the host scaffold is a selective and crucial criterion to the anion recognition. Our analyses reveal that coordinated charged metals provide a larger electrostatic stabilization to anion recognition, shifting the calixarenes cavity toward an electron deficient acidic character. This study contributes to the design and development of new organometallic macrocyclic hosts with increased anion recognition specificity.
中文翻译:

有机金属杯芳烃对阴离子的识别:相对论DFT计算的分析
在金属化杯[4]芳烃主体的背景下,利用Kohn-Sham分子轨道(KS-MO)理论,结合规范的能量分解分析,研究了参与阴离子识别的非共价相互作用的物理性质。校正的DFT理论水平。计算数据表明,最稳定的主体—客体结合发生在钌络合物主体中,其次是tech和钼金属化的大环受体。此外,客体在主支架中的空间配合是对阴离子识别的选择性和关键性标准。我们的分析表明,配位的带电金属为阴离子识别提供了更大的静电稳定性,使杯芳烃腔向电子不足的酸性特征移动。
更新日期:2018-06-27
中文翻译:

有机金属杯芳烃对阴离子的识别:相对论DFT计算的分析
在金属化杯[4]芳烃主体的背景下,利用Kohn-Sham分子轨道(KS-MO)理论,结合规范的能量分解分析,研究了参与阴离子识别的非共价相互作用的物理性质。校正的DFT理论水平。计算数据表明,最稳定的主体—客体结合发生在钌络合物主体中,其次是tech和钼金属化的大环受体。此外,客体在主支架中的空间配合是对阴离子识别的选择性和关键性标准。我们的分析表明,配位的带电金属为阴离子识别提供了更大的静电稳定性,使杯芳烃腔向电子不足的酸性特征移动。











































 京公网安备 11010802027423号
京公网安备 11010802027423号