Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational and Organizational Insights into Serum Proteins during Competitive Adsorption on Self-Assembled Monolayers
Langmuir ( IF 3.7 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.langmuir.8b01110 Abshar Hasan 1 , Gyan Waibhaw 1 , Lalit M. Pandey 1
Langmuir ( IF 3.7 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.langmuir.8b01110 Abshar Hasan 1 , Gyan Waibhaw 1 , Lalit M. Pandey 1
Affiliation
|
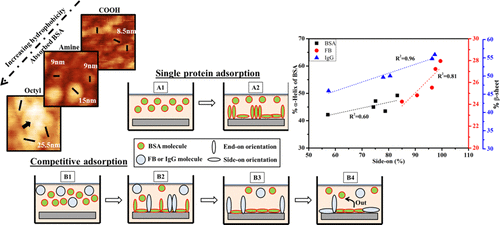
Physicochemical interactions of proteins with surfaces mediate the interactions between the implant and the biological system. Surface chemistry of the implant is crucial as it regulates the events at the interface. The objective of this study was to explore the performance of modified surfaces for such interactions relevant to various biomedical applications. Because of a wide range of surface wettability, we aimed to study protein behavior (i.e., conformational changes and their packing) during competitive protein adsorption. Three serum proteins (bovine serum albumin, BSA; fibrinogen, FB; and immunoglobulin G, IgG) were tested for their conformational changes and orientation upon adsorption on hydrophilic (COOH and amine), moderately hydrophobic (mixed and hybrid), and hydrophobic (octyl) surfaces generated via silanization. Modified surfaces were characterized using Fourier-transform infrared spectroscopy, contact angle, and atomic force microscopy (AFM) techniques. Adsorbed masses of proteins from single and binary protein solutions on different surfaces were quantified along with their secondary structure analyses. Maximum adsorbed protein masses were found to be on negatively charged and hydrophobic (octyl) surfaces because of ionic and hydrophobic interactions between protein molecules and surfaces, respectively. Side-on and end-on orientations of adsorbed protein molecules were analyzed using theoretical and AFM analyses. We observed compact and elongated forms of BSA molecules on hydrophilic and hydrophobic surfaces, respectively. We further found a linear increase in the α-helix content of BSA and β-sheet contents of FB and IgG proteins with the increasing side-on (%)-oriented protein molecules on the surfaces. This indicates that side-on orientations of adsorbed FB and IgG lead to the formation of β-sheets. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was employed to quantify the protein types and their ratio in competitively adsorbed proteins on different surfaces. A theoretical analysis was also used to determine the % secondary structures of competitively adsorbed proteins from BSA/FB and BSA/IgG solutions, which very well agreed with experimental results. The competitive protein adsorption from both BSA/FB and BSA/IgG solutions was found to be entropy-driven, as revealed by thermodynamic studies performed using isothermal titration calorimetry.
中文翻译:

在自组装单分子膜上竞争性吸附过程中血清蛋白的构象和组织洞察。
蛋白质与表面的物理化学相互作用介导了植入物与生物系统之间的相互作用。植入物的表面化学性质至关重要,因为它可以调节界面处的事件。这项研究的目的是探索改性表面在与各种生物医学应用相关的相互作用中的性能。由于表面润湿性范围广泛,我们旨在研究竞争性蛋白质吸附过程中的蛋白质行为(即构象变化及其堆积)。测试了三种血清蛋白(牛血清白蛋白,牛血清白蛋白,纤维蛋白原,FB和免疫球蛋白G,IgG)在亲水性(COOH和胺),中等疏水性(混合和杂化)和疏水性(辛基)上的构象变化和方向)通过硅烷化生成的表面。使用傅立叶变换红外光谱,接触角和原子力显微镜(AFM)技术对改性后的表面进行表征。定量分析了来自单一和二元蛋白质溶液在不同表面上的蛋白质吸附质量及其二级结构分析。由于分别在蛋白质分子和表面之间的离子和疏水相互作用,发现最大吸附的蛋白质质量在带负电荷的和疏水的(辛基)表面上。使用理论和原子力显微镜分析法分析了吸附的蛋白质分子的侧向和末端取向。我们分别在亲水性和疏水性表面上观察到BSA分子的紧密和细长形式。我们还发现,随着表面上侧向(%)定向蛋白分子的增加,BSA的α-螺旋含量以及FB和IgG蛋白的β-折叠含量呈线性增加。这表明吸附的FB和IgG的并排取向导致形成β-折叠。十二烷基硫酸钠聚丙烯酰胺凝胶电泳用于量化蛋白质类型及其在不同表面竞争性吸附蛋白质中的比例。还使用理论分析来确定BSA / FB和BSA / IgG溶液中竞争性吸附蛋白的二级结构%,这与实验结果非常吻合。使用等温滴定量热法进行的热力学研究表明,从BSA / FB和BSA / IgG溶液中吸收的竞争性蛋白质都是由熵驱动的。
更新日期:2018-06-25
中文翻译:

在自组装单分子膜上竞争性吸附过程中血清蛋白的构象和组织洞察。
蛋白质与表面的物理化学相互作用介导了植入物与生物系统之间的相互作用。植入物的表面化学性质至关重要,因为它可以调节界面处的事件。这项研究的目的是探索改性表面在与各种生物医学应用相关的相互作用中的性能。由于表面润湿性范围广泛,我们旨在研究竞争性蛋白质吸附过程中的蛋白质行为(即构象变化及其堆积)。测试了三种血清蛋白(牛血清白蛋白,牛血清白蛋白,纤维蛋白原,FB和免疫球蛋白G,IgG)在亲水性(COOH和胺),中等疏水性(混合和杂化)和疏水性(辛基)上的构象变化和方向)通过硅烷化生成的表面。使用傅立叶变换红外光谱,接触角和原子力显微镜(AFM)技术对改性后的表面进行表征。定量分析了来自单一和二元蛋白质溶液在不同表面上的蛋白质吸附质量及其二级结构分析。由于分别在蛋白质分子和表面之间的离子和疏水相互作用,发现最大吸附的蛋白质质量在带负电荷的和疏水的(辛基)表面上。使用理论和原子力显微镜分析法分析了吸附的蛋白质分子的侧向和末端取向。我们分别在亲水性和疏水性表面上观察到BSA分子的紧密和细长形式。我们还发现,随着表面上侧向(%)定向蛋白分子的增加,BSA的α-螺旋含量以及FB和IgG蛋白的β-折叠含量呈线性增加。这表明吸附的FB和IgG的并排取向导致形成β-折叠。十二烷基硫酸钠聚丙烯酰胺凝胶电泳用于量化蛋白质类型及其在不同表面竞争性吸附蛋白质中的比例。还使用理论分析来确定BSA / FB和BSA / IgG溶液中竞争性吸附蛋白的二级结构%,这与实验结果非常吻合。使用等温滴定量热法进行的热力学研究表明,从BSA / FB和BSA / IgG溶液中吸收的竞争性蛋白质都是由熵驱动的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号