当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nighttime Aqueous-Phase Formation of Nitrocatechols in the Atmospheric Condensed Phase
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-11 , DOI: 10.1021/acs.est.8b01161 Kristijan Vidović 1 , Damjan Lašič Jurković 2 , Martin Šala 1 , Ana Kroflič 1 , Irena Grgić 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-11 , DOI: 10.1021/acs.est.8b01161 Kristijan Vidović 1 , Damjan Lašič Jurković 2 , Martin Šala 1 , Ana Kroflič 1 , Irena Grgić 1
Affiliation
|
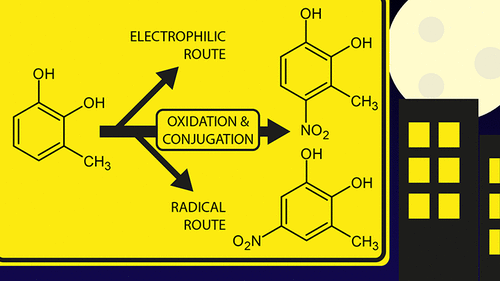
Yellow-colored methylnitrocatechols (MNC) contribute to the total organic aerosol mass and significantly alter absorption properties of the atmosphere. To date, their formation mechanisms are still not understood. In this work, the intriguing role of HNO2 (catalytic and oxidative) in the dark transformation of 3-methylcatechol (3MC) under atmospherically relevant aqueous-phase conditions is emphasized. Three possible pathways of dark 3-methyl-5-nitrocatechol and 3-methyl-4-nitrocatechol formation, markedly dependent on reaction conditions, were considered. In the dominant pathway, HNO2 is directly involved in the transformation of 3MC via consecutive oxidation and conjugated addition reactions (nonradical reaction mechanism). The two-step nitration dominates at a pH around the pKa of HNO2, which is typical for atmospheric aerosols, and is moderately dependent on temperature. Under very acidic conditions, the other two nitration pathways, oxidative aromatic nitration (electrophilic) and recombination of radical species, gain in importance. The predicted atmospheric lifetime of 3MC according to the dominant mechanism at these conditions (2.4 days at pH 4.5 and 25 °C) is more than 3-times shorter than that via the other two competitive pathways. Our results highlight the significance of a catechol oxidation-conjugated addition reaction in a nighttime secondary nitroaromatic chromophore formation in the atmosphere, especially in polluted environments with high NOx concentrations and relatively acidic particles (pH around 3).
中文翻译:

大气冷凝阶段硝基邻苯二酚的夜间水相形成
黄色的甲基硝基邻苯二酚(MNC)有助于增加有机气溶胶的总量,并显着改变大气的吸收特性。迄今为止,它们的形成机理仍不为人所知。在这项工作中,强调了在与大气有关的水相条件下,HNO 2(催化和氧化)在3-甲基邻苯二酚(3MC)的黑暗转化中的有趣作用。考虑了暗的3-甲基-5-硝基邻苯二酚和3-甲基-4-硝基邻苯二酚形成的三种可能途径,这些途径明显取决于反应条件。在主要途径中,HNO 2通过连续的氧化和共轭加成反应(非自由基反应机理)直接参与3MC的转化。两步硝化反应在pH值约为p时占主导地位。HNO 2的K a是大气气溶胶的典型特征,适度取决于温度。在非常酸性的条件下,其他两个硝化途径,即氧化性芳香族硝化(亲电子的)和自由基种类的重组,变得尤为重要。在这些条件下(pH 4.5和25°C下2.4天),根据主要机理预测的3MC大气寿命比通过其他两种竞争途径的寿命短3倍以上。我们的研究结果突出显示在大气中的夜间次级硝基芳族发色团的形成儿茶酚氧化偶联加成反应的意义,特别是在具有高NO污染环境X浓度和相对酸性的粒子(pH约3)。
更新日期:2018-07-12
中文翻译:

大气冷凝阶段硝基邻苯二酚的夜间水相形成
黄色的甲基硝基邻苯二酚(MNC)有助于增加有机气溶胶的总量,并显着改变大气的吸收特性。迄今为止,它们的形成机理仍不为人所知。在这项工作中,强调了在与大气有关的水相条件下,HNO 2(催化和氧化)在3-甲基邻苯二酚(3MC)的黑暗转化中的有趣作用。考虑了暗的3-甲基-5-硝基邻苯二酚和3-甲基-4-硝基邻苯二酚形成的三种可能途径,这些途径明显取决于反应条件。在主要途径中,HNO 2通过连续的氧化和共轭加成反应(非自由基反应机理)直接参与3MC的转化。两步硝化反应在pH值约为p时占主导地位。HNO 2的K a是大气气溶胶的典型特征,适度取决于温度。在非常酸性的条件下,其他两个硝化途径,即氧化性芳香族硝化(亲电子的)和自由基种类的重组,变得尤为重要。在这些条件下(pH 4.5和25°C下2.4天),根据主要机理预测的3MC大气寿命比通过其他两种竞争途径的寿命短3倍以上。我们的研究结果突出显示在大气中的夜间次级硝基芳族发色团的形成儿茶酚氧化偶联加成反应的意义,特别是在具有高NO污染环境X浓度和相对酸性的粒子(pH约3)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号