当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biophysical Examination of the Calcium-Modulated Nickel-Binding Properties of Human Calprotectin Reveals Conformational Change in the EF-Hand Domains and His3Asp Site
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acs.biochem.8b00415 Toshiki G. Nakashige , Sarah E. J. Bowman , Emily M. Zygiel , Catherine L. Drennan , Elizabeth M. Nolan
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-11 00:00:00 , DOI: 10.1021/acs.biochem.8b00415 Toshiki G. Nakashige , Sarah E. J. Bowman , Emily M. Zygiel , Catherine L. Drennan , Elizabeth M. Nolan
|
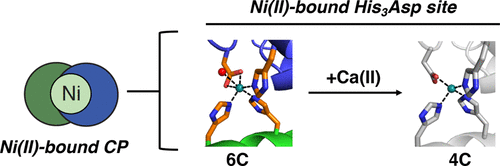
Calprotectin (CP, S100A8/S100A9 oligomer, MRP-8/MRP-14 oligomer) is a host-defense protein that sequesters nutrient transition metals from microbes. Each S100A8/S100A9 heterodimer contains four EF-hand domains and two transition-metal-binding sites. We investigate the effect of Ca(II) ions on the structure and Ni(II)-binding properties of human CP. By employing energy dispersive X-ray (EDX) spectroscopy, we evaluate the metal content of Ni(II)-bound CP-Ser [oligomer of S100A8(C42S) and S100A9(C3S)] crystals obtained in the absence and presence of Ca(II). We present a 2.1 Å resolution crystal structure of Ni(II)-bound CP-Ser and compare this structure to a reported Ni(II)- and Ca(II)-bound CP-Ser structure [Nakashige, T. G., et al. (2017) J. Am. Chem. Soc.139, 8828–8836]. This analysis reveals conformational changes associated with coordination of Ca(II) to the EF-hands of S100A9 and that Ca(II) binding affects the coordination number and geometry of the Ni(II) ion bound to the His3Asp site. In contrast, negligible differences are observed for the Ni(II)-His6 site in the absence and presence of Ca(II). Biochemical studies show that, whereas the His6 site has a thermodynamic preference for Ni(II) over Zn(II), the His3Asp site selects for Zn(II) over Ni(II), and relatively rapid metal exchange occurs at this site. These observations inform the working model for how CP withholds nutrient metals in the extracellular space.
中文翻译:

钙调节的镍钙结合蛋白的人类钙卫蛋白的生物物理检查揭示了构象变化的EF手域和他的3天冬氨酸位点。
钙卫蛋白(钙卫蛋白,CP,S100A8 / S100A9低聚物,MRP-8 / MRP-14低聚物)是一种宿主防御蛋白,可以隔离微生物中的营养过渡金属。每个S100A8 / S100A9异二聚体包含四个EF-手结构域和两个过渡金属结合位点。我们调查了Ca(II)离子对人CP结构和Ni(II)结合特性的影响。通过使用能量色散X射线(EDX)光谱,我们评估了在不存在和存在Ca( II)。我们提出了Ni(II)结合的CP-Ser的2.1Å分辨率晶体结构,并将这种结构与已报道的Ni(II)和Ca(II)结合的CP-Ser结构进行了比较[Nakashige,TG等。(2017)J.Am. 化学 Soc。139,8828–8836]。该分析揭示了与Ca(II)与S100A9的EF手配位相关的构象变化,并且Ca(II)的结合会影响结合到His 3 Asp位点的Ni(II)离子的配位数和几何形状。相反,在不存在和存在Ca(II)的情况下,观察到Ni(II)-His 6位点的差异可忽略不计。生化研究表明,尽管His 6位点对Ni(II)的热力学偏好高于Zn(II),但是His 3 Asp位点对Zn(II)的选择高于Ni(II),并且在此发生较快速的金属交换地点。这些观察结果为CP如何在细胞外空间保留营养金属提供了工作模型。
更新日期:2018-06-11
中文翻译:

钙调节的镍钙结合蛋白的人类钙卫蛋白的生物物理检查揭示了构象变化的EF手域和他的3天冬氨酸位点。
钙卫蛋白(钙卫蛋白,CP,S100A8 / S100A9低聚物,MRP-8 / MRP-14低聚物)是一种宿主防御蛋白,可以隔离微生物中的营养过渡金属。每个S100A8 / S100A9异二聚体包含四个EF-手结构域和两个过渡金属结合位点。我们调查了Ca(II)离子对人CP结构和Ni(II)结合特性的影响。通过使用能量色散X射线(EDX)光谱,我们评估了在不存在和存在Ca( II)。我们提出了Ni(II)结合的CP-Ser的2.1Å分辨率晶体结构,并将这种结构与已报道的Ni(II)和Ca(II)结合的CP-Ser结构进行了比较[Nakashige,TG等。(2017)J.Am. 化学 Soc。139,8828–8836]。该分析揭示了与Ca(II)与S100A9的EF手配位相关的构象变化,并且Ca(II)的结合会影响结合到His 3 Asp位点的Ni(II)离子的配位数和几何形状。相反,在不存在和存在Ca(II)的情况下,观察到Ni(II)-His 6位点的差异可忽略不计。生化研究表明,尽管His 6位点对Ni(II)的热力学偏好高于Zn(II),但是His 3 Asp位点对Zn(II)的选择高于Ni(II),并且在此发生较快速的金属交换地点。这些观察结果为CP如何在细胞外空间保留营养金属提供了工作模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号