当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of D1-V185 on the Water Molecules That Facilitate O2 Formation by the Catalytic Mn4CaO5 Cluster in Photosystem II
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.biochem.8b00630 Christopher J. Kim 1 , Han Bao 2 , Robert L. Burnap 2 , Richard J. Debus 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.biochem.8b00630 Christopher J. Kim 1 , Han Bao 2 , Robert L. Burnap 2 , Richard J. Debus 1
Affiliation
|
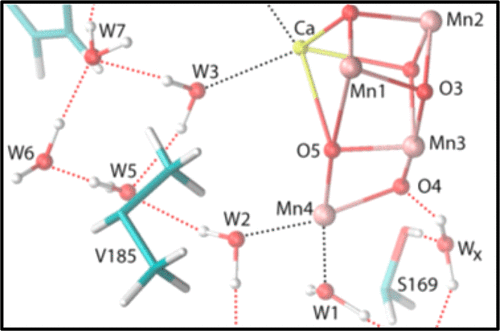
The oxidations of the O2-evolving Mn4CaO5 cluster in Photosystem II are coupled to the release of protons to the thylakoid lumen via one or more proton egress pathways. These pathways are comprised of extensive networks of hydrogen-bonded water molecules and amino acid side chains. The hydrophobic residue, D1-V185, is adjacent to numerous water molecules in one of these pathways. The D1-V185N mutation dramatically slows O–O bond formation. This impairment has been attributed to a disruption of the hydrogen-bonded water molecules that are crucial for proton egress or whose rearrangement is required for catalysis. In this study, Fourier transform infrared spectroscopy was employed to characterize the impact of the D1-V185N mutation on the carboxylate groups and water molecules that form a network of hydrogen bonds in this putative proton egress pathway. By analyzing carboxylate stretching modes, carbonyl stretching modes of hydrogen-bonded carboxylic acids, O–H stretching modes of hydrogen-bonded water molecules, and D–O–D bending modes, we obtain evidence that the D1-V185N mutation perturbs the extensive network of hydrogen bonds that extends from YZ to D1-D61 to a greater extent than any mutation yet examined but does not alter the water molecules that interact directly with D1-D61. The mutation also alters the environments of the carboxylate groups whose pKa values change in response to the S1 to S2 and S2 to S3 transitions. Finally, the mutation alters the environment of the water molecule whose bending mode vanishes during the S2 to S3 transition, consistent with assigning the Ca2+-bound W3 as the water molecule that deprotonates and joins oxo bridge O5 during the S2 to S3 transition, possibly as the second substrate water molecule for O2 formation.
中文翻译:

D1-V185对光系统II中催化Mn 4 CaO 5团簇促进O 2形成的水分子的影响
析出O 2的Mn 4 CaO 5的氧化Photosystem II中的团簇通过一个或多个质子流出途径与质子向类囊体腔的释放耦合。这些途径由氢键水分子和氨基酸侧链的广泛网络组成。疏水残基D1-V185在这些途径之一中与众多水分子相邻。D1-V185N突变会大大减慢O-O键的形成。这种损害归因于氢键结合的水分子的破坏,这对于质子逸出或催化需要重排是至关重要的。在这项研究中,采用傅立叶变换红外光谱法来表征D1-V185N突变对在该假定的质子逸出途径中形成氢键网络的羧酸酯基团和水分子的影响。Z到D1-D61的程度比尚未检查的任何突变都大,但不会改变直接与D1-D61相互作用的水分子。突变还改变了其羧酸盐基团的环境,其p K a值响应于S 1至S 2和S 2至S 3的转变而变化。最后,该突变改变了水分子,其弯曲模式在S期间消失的环境2至S 3的过渡,与分配的Ca一致2+结合的W3随着水分子去质子化和S中加入氧代桥O5 2至小号3过渡,可能作为形成O 2的第二种底物水分子。
更新日期:2018-06-26
中文翻译:

D1-V185对光系统II中催化Mn 4 CaO 5团簇促进O 2形成的水分子的影响
析出O 2的Mn 4 CaO 5的氧化Photosystem II中的团簇通过一个或多个质子流出途径与质子向类囊体腔的释放耦合。这些途径由氢键水分子和氨基酸侧链的广泛网络组成。疏水残基D1-V185在这些途径之一中与众多水分子相邻。D1-V185N突变会大大减慢O-O键的形成。这种损害归因于氢键结合的水分子的破坏,这对于质子逸出或催化需要重排是至关重要的。在这项研究中,采用傅立叶变换红外光谱法来表征D1-V185N突变对在该假定的质子逸出途径中形成氢键网络的羧酸酯基团和水分子的影响。Z到D1-D61的程度比尚未检查的任何突变都大,但不会改变直接与D1-D61相互作用的水分子。突变还改变了其羧酸盐基团的环境,其p K a值响应于S 1至S 2和S 2至S 3的转变而变化。最后,该突变改变了水分子,其弯曲模式在S期间消失的环境2至S 3的过渡,与分配的Ca一致2+结合的W3随着水分子去质子化和S中加入氧代桥O5 2至小号3过渡,可能作为形成O 2的第二种底物水分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号