当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Allosteric Activation Shifts the Rate-Limiting Step in a Short-Form ATP Phosphoribosyltransferase
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.biochem.8b00559 Gemma Fisher 1 , Catherine M. Thomson 1 , Rozanne Stroek 1 , Clarissa M. Czekster 1 , Jennifer S. Hirschi 2 , Rafael G. da Silva 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.biochem.8b00559 Gemma Fisher 1 , Catherine M. Thomson 1 , Rozanne Stroek 1 , Clarissa M. Czekster 1 , Jennifer S. Hirschi 2 , Rafael G. da Silva 1
Affiliation
|
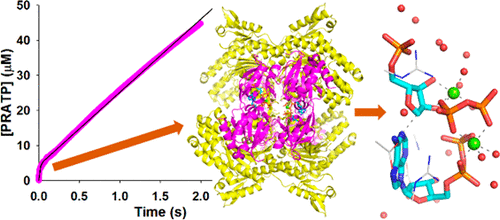
Short-form ATP phosphoribosyltransferase (ATPPRT) is a hetero-octameric allosteric enzyme comprising four catalytic subunits (HisGS) and four regulatory subunits (HisZ). ATPPRT catalyzes the Mg2+-dependent condensation of ATP and 5-phospho-α-d-ribosyl-1-pyrophosphate (PRPP) to generate N1-(5-phospho-β-d-ribosyl)-ATP (PRATP) and pyrophosphate, the first reaction of histidine biosynthesis. While HisGS is catalytically active on its own, its activity is allosterically enhanced by HisZ in the absence of histidine. In the presence of histidine, HisZ mediates allosteric inhibition of ATPPRT. Here, initial velocity patterns, isothermal titration calorimetry, and differential scanning fluorimetry establish a distinct kinetic mechanism for ATPPRT where PRPP is the first substrate to bind. AMP is an inhibitor of HisGS, but steady-state kinetics and 31P NMR spectroscopy demonstrate that ADP is an alternative substrate. Replacement of Mg2+ by Mn2+ enhances catalysis by HisGS but not by the holoenzyme, suggesting different rate-limiting steps for nonactivated and activated enzyme forms. Density functional theory calculations posit an SN2-like transition state stabilized by two equivalents of the metal ion. Natural bond orbital charge analysis points to Mn2+ increasing HisGS reaction rate via more efficient charge stabilization at the transition state. High solvent viscosity increases HisGS’s catalytic rate, but decreases the hetero-octamer’s, indicating that chemistry and product release are rate-limiting for HisGS and ATPPRT, respectively. This is confirmed by pre-steady-state kinetics, with a burst in product formation observed with the hetero-octamer but not with HisGS. These results are consistent with an activation mechanism whereby HisZ binding leads to a more active conformation of HisGS, accelerating chemistry beyond the product release rate.
中文翻译:

变构活化改变了短型ATP磷酸核糖基转移酶中的限速步骤
短形式的ATP磷酸(ATPPRT)是包括四个催化亚基(HisG杂八聚体变构酶小号)和四个调节亚基(HisZ)。ATPPRT催化Mg 2+依赖性的ATP与5-磷酸-α- d-核糖基-1-焦磷酸(PRPP)的缩合生成N 1-(5-磷酸-β - d-核糖基)-ATP(PRATP)和焦磷酸,组氨酸生物合成的第一个反应。而His G S它本身具有催化活性,在没有组氨酸的情况下,HisZ会变构地增强其活性。在组氨酸存在下,HisZ介导ATPPRT的变构抑制。在这里,初始速度模式,等温滴定量热法和差示扫描荧光法建立了ATPPRT独特的动力学机制,其中PRPP是第一个结合的底物。AMP是HisG的抑制剂小号,但稳态动力学和31 P NMR光谱表明ADP是一种替代衬底。的Mg替换2+用Mn 2+通过HisG增强催化小号但不是通过全酶,这表明非活化和活化酶形式的限速步骤不同。密度泛函理论计算假定了由两当量的金属离子稳定的类似S N 2的过渡态。自然键轨道电荷分析点与Mn 2+增加HisG小号通过更有效的电荷稳定在过渡态的反应速率。高的溶剂的粘度增加HisG小号的催化速率,但是降低了杂八聚体的,这表明化学和产品释放是限速HisG小号分别和ATPPRT。前稳态动力学证实了这一点,在杂八聚体中观察到产物形成突然爆发,而HisG则未观察到小号。这些结果与激活机制,使HisZ结合导致HisG更积极的构象一致的小号,加速化学反应超出了产品的发布速度。
更新日期:2018-06-25
中文翻译:

变构活化改变了短型ATP磷酸核糖基转移酶中的限速步骤
短形式的ATP磷酸(ATPPRT)是包括四个催化亚基(HisG杂八聚体变构酶小号)和四个调节亚基(HisZ)。ATPPRT催化Mg 2+依赖性的ATP与5-磷酸-α- d-核糖基-1-焦磷酸(PRPP)的缩合生成N 1-(5-磷酸-β - d-核糖基)-ATP(PRATP)和焦磷酸,组氨酸生物合成的第一个反应。而His G S它本身具有催化活性,在没有组氨酸的情况下,HisZ会变构地增强其活性。在组氨酸存在下,HisZ介导ATPPRT的变构抑制。在这里,初始速度模式,等温滴定量热法和差示扫描荧光法建立了ATPPRT独特的动力学机制,其中PRPP是第一个结合的底物。AMP是HisG的抑制剂小号,但稳态动力学和31 P NMR光谱表明ADP是一种替代衬底。的Mg替换2+用Mn 2+通过HisG增强催化小号但不是通过全酶,这表明非活化和活化酶形式的限速步骤不同。密度泛函理论计算假定了由两当量的金属离子稳定的类似S N 2的过渡态。自然键轨道电荷分析点与Mn 2+增加HisG小号通过更有效的电荷稳定在过渡态的反应速率。高的溶剂的粘度增加HisG小号的催化速率,但是降低了杂八聚体的,这表明化学和产品释放是限速HisG小号分别和ATPPRT。前稳态动力学证实了这一点,在杂八聚体中观察到产物形成突然爆发,而HisG则未观察到小号。这些结果与激活机制,使HisZ结合导致HisG更积极的构象一致的小号,加速化学反应超出了产品的发布速度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号