当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Large-Scale Assessment of Extractables and Leachables in Single-Use Bags for Biomanufacturing
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.analchem.8b01208 Noemí Dorival-García 1 , Sara Carillo 1 , Christine Ta 1 , Dominic Roberts 2 , Kate Comstock 3 , Simon Lofthouse 4 , Elena Ciceri 5 , Kyle D’Silva 4 , Gerald Kierans 6 , Christian Kaisermayer 7 , Anna Lindeberg 7 , Jonathan Bones 1, 8
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1021/acs.analchem.8b01208 Noemí Dorival-García 1 , Sara Carillo 1 , Christine Ta 1 , Dominic Roberts 2 , Kate Comstock 3 , Simon Lofthouse 4 , Elena Ciceri 5 , Kyle D’Silva 4 , Gerald Kierans 6 , Christian Kaisermayer 7 , Anna Lindeberg 7 , Jonathan Bones 1, 8
Affiliation
|
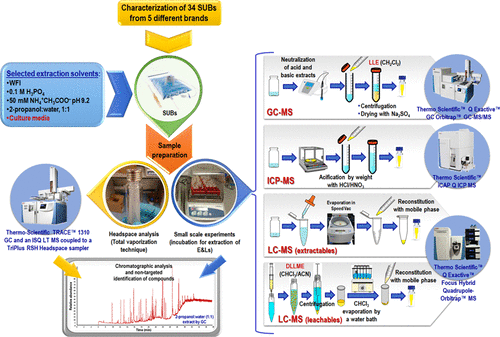
Single-use technologies (SUTs) are widely used during biopharmaceutical manufacture as disposable bioreactors or media and buffer storage bags. Despite their advantages, the risk of release of extractable and leachable (E&Ls) substances is considered an important drawback in adopting disposables in the biomanufacturing process. E&Ls may detrimentally affect cell viability or productivity or may persist during purification and present a risk to the patient if remaining in the final drug product. In this study, 34 plastic films from single-use bags (SUBs) for cell cultivation were extracted with selected solvents that represent reasonable worst-case conditions for most typical biomanufacturing applications. SUBs were incubated at small-scale under accelerated-aging conditions that represented standard operational conditions of use. Leachables analysis was performed following dispersive liquid–liquid microextraction (DLLME) for analyte preconcentration and removal of matrix interference. Resulting extracts were characterized by GC-headspace for volatiles, high resolution GC-Orbitrap-MS/MS for semivolatiles, high resolution LC-Orbitrap-MS/MS for nonvolatiles, and ICP-MS for trace elemental analysis. Multivariate statistical analysis of the analytical data revealed significant correlations between the type and concentration of compounds and bags features including brand, manufacturing date and polymer type. The analytical data demonstrates that, over recent years, the nature of E&Ls has been altered due to the implementation of manufacturing changes and new types of polymers and may change further with the future advent of regulations that will limit or ban the use of certain raw materials and additives. The broad E&L database generated herein facilitates toxicological assessments from a biomanufacturing standpoint and provides practical guidelines for confident determination of E&Ls to enable screening and elimination of nonsatisfactory films for single use bioprocessing.
中文翻译:

一次性评估用于生物制造的一次性袋子中的可萃取物和可浸出物的大规模评估
一次性技术(SUT)在生物制药生产中被广泛用作一次性生物反应器或培养基和缓冲液存储袋。尽管具有优势,但在生物制造过程中采用一次性用品时,释放可萃取和可浸出(E&Ls)物质的风险被认为是一个重要的缺点。E&L可能有害地影响细胞活力或生产率,或者在纯化过程中可能持续存在,如果保留在最终药物产品中,则可能给患者带来风险。在这项研究中,用选定的溶剂提取了一次性袋(SUBs)中用于细胞培养的34层塑料薄膜,这些溶剂代表了最典型的生物制造应用中最合理的最坏情况。SUBs在代表使用标准操作条件的加速老化条件下进行了小规模温育。分散液-液微萃取(DLLME)后进行可浸出物分析,以进行分析物预浓缩和消除基质干扰。所得提取物的特征在于:用于挥发性物质的GC顶空,用于半挥发性物质的高分辨率GC-Orbitrap-MS / MS,用于非挥发性物质的高分辨率LC-Orbitrap-MS / MS和用于痕量元素分析的ICP-MS。分析数据的多变量统计分析显示,化合物的类型和浓度与袋子特征(包括品牌,生产日期和聚合物类型)之间存在显着相关性。分析数据表明,近年来,E&由于制造变更和新型聚合物的实施,Ls已被更改,并且随着将来限制或禁止使用某些原材料和添加剂的法规的出现,Ls可能会进一步改变。本文生成的广泛的E&L数据库有助于从生物制造的角度进行毒理学评估,并为确定E&L的可靠确定性提供实用指南,从而能够筛选和消除一次性生物处理过程中不满意的胶片。
更新日期:2018-06-26
中文翻译:

一次性评估用于生物制造的一次性袋子中的可萃取物和可浸出物的大规模评估
一次性技术(SUT)在生物制药生产中被广泛用作一次性生物反应器或培养基和缓冲液存储袋。尽管具有优势,但在生物制造过程中采用一次性用品时,释放可萃取和可浸出(E&Ls)物质的风险被认为是一个重要的缺点。E&L可能有害地影响细胞活力或生产率,或者在纯化过程中可能持续存在,如果保留在最终药物产品中,则可能给患者带来风险。在这项研究中,用选定的溶剂提取了一次性袋(SUBs)中用于细胞培养的34层塑料薄膜,这些溶剂代表了最典型的生物制造应用中最合理的最坏情况。SUBs在代表使用标准操作条件的加速老化条件下进行了小规模温育。分散液-液微萃取(DLLME)后进行可浸出物分析,以进行分析物预浓缩和消除基质干扰。所得提取物的特征在于:用于挥发性物质的GC顶空,用于半挥发性物质的高分辨率GC-Orbitrap-MS / MS,用于非挥发性物质的高分辨率LC-Orbitrap-MS / MS和用于痕量元素分析的ICP-MS。分析数据的多变量统计分析显示,化合物的类型和浓度与袋子特征(包括品牌,生产日期和聚合物类型)之间存在显着相关性。分析数据表明,近年来,E&由于制造变更和新型聚合物的实施,Ls已被更改,并且随着将来限制或禁止使用某些原材料和添加剂的法规的出现,Ls可能会进一步改变。本文生成的广泛的E&L数据库有助于从生物制造的角度进行毒理学评估,并为确定E&L的可靠确定性提供实用指南,从而能够筛选和消除一次性生物处理过程中不满意的胶片。











































 京公网安备 11010802027423号
京公网安备 11010802027423号