当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclic Regulation of the Sulfilimine Bond in Peptides and NC1 Hexamers via the HOBr/H2Se Conjugated System
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.analchem.8b02228 Dongrui Luan 1 , Xiaonan Gao 1 , Fanpeng Kong 1 , Xiaoxiao Song 1 , Aishan Zheng 1 , Xiaojun Liu 1 , Kehua Xu 1 , Bo Tang 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.analchem.8b02228 Dongrui Luan 1 , Xiaonan Gao 1 , Fanpeng Kong 1 , Xiaoxiao Song 1 , Aishan Zheng 1 , Xiaojun Liu 1 , Kehua Xu 1 , Bo Tang 1
Affiliation
|
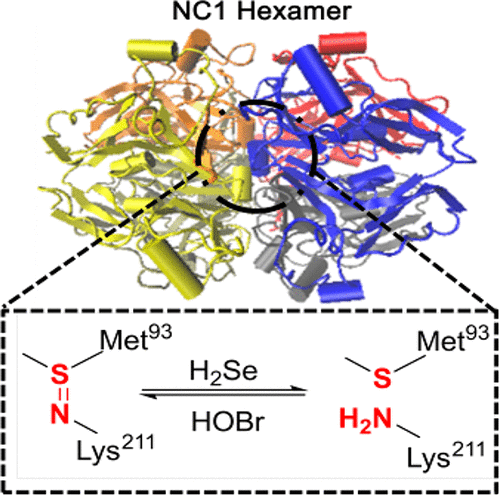
The sulfilimine bond (−S═N−), found in the collagen IV scaffold, significantly stabilizes the architecture via the formation of sulfilimine cross-links. However, precisely governing the formation and breakup process of the sulfilimine bond in living organisms for better life functions still remains a challenge. Hence, we established a new way to regulate the breaking and formation of the sulfilimine bond through hydrogen selenide (H2Se) and hypobromous acid (HOBr), which can be easily controlled at simulated physiological conditions. This novel strategy provides a circulation regulation system to modulate the sulfilimine bond in peptides and NC1 hexamers, which can offer a substantial system for further study of the physiological function of collagen IV.
中文翻译:

通过HOBr / H 2 Se共轭体系对肽和NC1六聚体中亚硫胺键的循环调节
在胶原蛋白IV支架中发现的亚硫胺键(-S═N-)通过形成亚硫胺交联键来显着稳定体系结构。然而,精确控制生物体中亚硫亚胺键的形成和分解过程以实现更好的生命功能仍然是一个挑战。因此,我们建立了一种通过硒化氢(H 2 Se)和次溴酸(HOBr)调节硫亚胺键断裂和形成的新方法,该方法可以在模拟的生理条件下轻松控制。这种新颖的策略提供了一种循环调节系统,可调节肽和NC1六聚体中的亚硫胺键,这可为进一步研究胶原IV的生理功能提供一个实质性的系统。
更新日期:2018-06-25
中文翻译:

通过HOBr / H 2 Se共轭体系对肽和NC1六聚体中亚硫胺键的循环调节
在胶原蛋白IV支架中发现的亚硫胺键(-S═N-)通过形成亚硫胺交联键来显着稳定体系结构。然而,精确控制生物体中亚硫亚胺键的形成和分解过程以实现更好的生命功能仍然是一个挑战。因此,我们建立了一种通过硒化氢(H 2 Se)和次溴酸(HOBr)调节硫亚胺键断裂和形成的新方法,该方法可以在模拟的生理条件下轻松控制。这种新颖的策略提供了一种循环调节系统,可调节肽和NC1六聚体中的亚硫胺键,这可为进一步研究胶原IV的生理功能提供一个实质性的系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号