当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembly of α-Tocopherol Transfer Protein Nanoparticles: A Patchy Protein Model
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-11 , DOI: 10.1021/acs.jpcb.8b05936 Raphael Mathias Peltzer 1 , Hima Bindu Kolli 1 , Achim Stocker 2 , Michele Cascella 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-11 , DOI: 10.1021/acs.jpcb.8b05936 Raphael Mathias Peltzer 1 , Hima Bindu Kolli 1 , Achim Stocker 2 , Michele Cascella 1
Affiliation
|
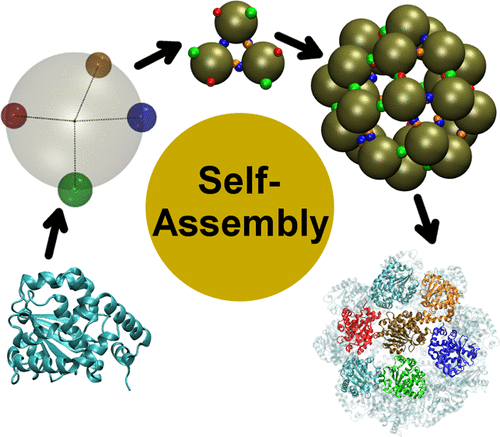
We describe the mechanism of self-aggregation of α-tocopherol transfer protein into a spherical nanocage employing Monte Carlo simulations. The protein is modeled by a patchy coarse-grained representation, where the protein–protein interfaces, determined in the past by X-ray diffraction, are represented by simplified two-body interaction potentials. Our results show that the oligomerization kinetics proceeds in two steps, with the formation of metastable trimeric units and the subsequent assembly into the spherical aggregates. Data are in agreement with experimental observations regarding the prevalence of different aggregation states at specific ambient conditions. Finally, our results indicate a route for the experimental stabilization of the trimer, crucial for the understanding of the physiological role of such aggregates in vitamin E body trafficking.
中文翻译:

α-生育酚转移蛋白纳米粒子的自组装:修补蛋白模型。
我们描述了使用蒙特卡洛模拟法将α-生育酚转移蛋白自我聚集到球形纳米笼中的机制。蛋白质是用斑驳的粗粒表示法建模的,过去通过X射线衍射确定的蛋白质-蛋白质界面以简化的两体相互作用势来表示。我们的结果表明,低聚动力学分两个步骤进行,形成亚稳的三聚体单元,然后组装成球形聚集体。数据与关于在特定环境条件下不同聚集状态的普遍性的实验观察结果一致。最后,我们的结果表明了三聚体实验稳定的途径,
更新日期:2018-07-12
中文翻译:

α-生育酚转移蛋白纳米粒子的自组装:修补蛋白模型。
我们描述了使用蒙特卡洛模拟法将α-生育酚转移蛋白自我聚集到球形纳米笼中的机制。蛋白质是用斑驳的粗粒表示法建模的,过去通过X射线衍射确定的蛋白质-蛋白质界面以简化的两体相互作用势来表示。我们的结果表明,低聚动力学分两个步骤进行,形成亚稳的三聚体单元,然后组装成球形聚集体。数据与关于在特定环境条件下不同聚集状态的普遍性的实验观察结果一致。最后,我们的结果表明了三聚体实验稳定的途径,











































 京公网安备 11010802027423号
京公网安备 11010802027423号