当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dependence of the Formation of Tau and Aβ Peptide Mixed Aggregates on the Secondary Structure of the N-Terminal Region of Aβ
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.jpcb.8b04647 Ana V. Rojas 1 , Gia G. Maisuradze 2 , Harold A. Scheraga 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.jpcb.8b04647 Ana V. Rojas 1 , Gia G. Maisuradze 2 , Harold A. Scheraga 2
Affiliation
|
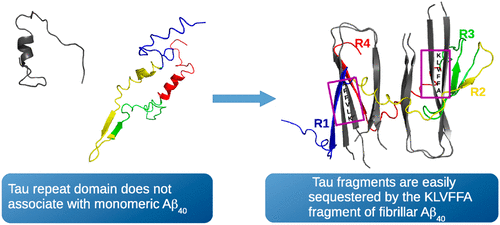
One of the hallmarks of Alzheimer’s disease is the formation of aggregates of the tau protein, a process that can be facilitated by the presence of fibrils formed by the amyloid β peptide (Aβ). However, the mechanism that triggers tau aggregation is still a matter of debate. The effect of Aβ40 fibrils on the aggregation of the repeat domain of tau (TauRD) is investigated here by employing coarse-grained molecular dynamics simulations. The results indicate that the repeat domain of tau has a high affinity for Aβ40 fibrils, with the 261GSTENLK267 fragment of tau driving TauRD toward the 16KLVFFA21 fragment in Aβ40. Monomeric Aβ40, in which the 16KLVFFA21 fragment is rarely found in an extended conformation (as in the fibril), has a low affinity for the TauRD, indicating that the ability of Aβ40 fibrils to bind to the TauRD depends on the 16KLVFFA21 fragment of Aβ adopting an extended conformation.
中文翻译:

Tau和Aβ肽混合聚集体的形成对AβN末端区域二级结构的依赖性
阿尔茨海默氏病的标志之一是tau蛋白聚集体的形成,这一过程可通过存在由淀粉样β肽(Aβ)形成的原纤维来促进。但是,触发tau聚集的机制仍是一个争论的问题。Aβ的影响40原纤维上tau的重复结构域(TauRD)的聚合是通过使用粗粒度的分子动力学模拟这里调查。结果表明,tau的重复结构域具有用于Aβ的亲和性高40原纤维,具有261 GSTENLK 267的tau驱动TauRD朝向的片段16 KLVFFA 21在Aβ片段40。单体Aβ40,其中在扩展构象中很少发现16 KLVFFA 21片段(如在原纤维中),对TauRD的亲和力低,表明Aβ40纤维与TauRD结合的能力取决于16 KLVFFA 21片段的Aβ采用扩展构象。
更新日期:2018-07-12
中文翻译:

Tau和Aβ肽混合聚集体的形成对AβN末端区域二级结构的依赖性
阿尔茨海默氏病的标志之一是tau蛋白聚集体的形成,这一过程可通过存在由淀粉样β肽(Aβ)形成的原纤维来促进。但是,触发tau聚集的机制仍是一个争论的问题。Aβ的影响40原纤维上tau的重复结构域(TauRD)的聚合是通过使用粗粒度的分子动力学模拟这里调查。结果表明,tau的重复结构域具有用于Aβ的亲和性高40原纤维,具有261 GSTENLK 267的tau驱动TauRD朝向的片段16 KLVFFA 21在Aβ片段40。单体Aβ40,其中在扩展构象中很少发现16 KLVFFA 21片段(如在原纤维中),对TauRD的亲和力低,表明Aβ40纤维与TauRD结合的能力取决于16 KLVFFA 21片段的Aβ采用扩展构象。











































 京公网安备 11010802027423号
京公网安备 11010802027423号