当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Glutamine-Rich Carrier Efficiently Delivers Anti-CD47 siRNA Driven by a “Glutamine Trap” To Inhibit Lung Cancer Cell Growth
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00076 Jiamin Wu 1 , Zhi Li 1 , Zeping Yang 1 , Ling Guo 1 , Ye Zhang 1 , Huihui Deng 1 , Cuifeng Wang 1 , Min Feng 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00076 Jiamin Wu 1 , Zhi Li 1 , Zeping Yang 1 , Ling Guo 1 , Ye Zhang 1 , Huihui Deng 1 , Cuifeng Wang 1 , Min Feng 1
Affiliation
|
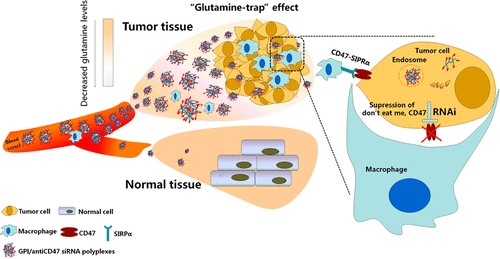
It is not efficient enough using the current approaches for tumor-selective drug delivery based on the EPR effect and ligand–receptor interactions, and they have largely failed to translate into the clinic. Therefore, it is urgent to explore an enhanced strategy for effective delivery of anticancer agents. Clinically, many cancers require large amounts of glutamine for their continued growth and survival, resulting in circulating glutamine extraction by the tumor being much greater than that for any organs, behaving as a “glutamine trap”. In the present study, we sought to elucidate whether the glutamine-trap effect could be exploited to deliver therapeutic agents to selectively kill cancer cells. Here, a macromolecular glutamine analogue, glutamine-functionalized branched polyethylenimine (GPI), was constructed as the carrier to deliver anti-CD47 siRNA for the blockage of CD47 “don’t eat me” signals on cancer cells. The GPI/siRNA glutamine-rich polyplexes exhibited remarkably high levels of cellular uptake by glutamine-dependent lung cancer cells, wild-type A549 cells (A549WT), and its cisplatin-resistant cells (A549DDP), specifically under glutamine-depleted conditions. It was noted that the glutamine transporter ASCT2 was highly expressed both on A549WT and A549DDP but with almost no expression in normal human lung fibroblasts cells. Inhibition of ASCT2 significantly prevented the internalization of GPI polyplexes. These findings raised the intriguing possibility that the glutamine-rich GPI polyplexes utilize the ASCT2 pathway to selectively facilitate their cellular uptake by cancer cells. GPI further delivered anti-CD47 siRNA efficiently both in vitro and in vivo to downregulate the intratumoral mRNA and protein expression levels of CD47. CD47 functions as a “don’t eat me” signal and binds to the immunoreceptor SIRPα inducing evasion of phagocytic clearance. GPI/anti-CD47 siRNA polyplexes achieved significant antitumor activities both on A549WT and A549DDP tumor-bearing nude mice. Notably, it had no adverse effect on CD47-expressing red blood cells and platelets, likely because of selective delivery. Therefore, the glutamine-rich carrier GPI driven by the glutamine-trap effect provides a promising new strategy for designing anticancer drug delivery systems.
中文翻译:

富含谷氨酰胺的载体可有效提供“谷氨酰胺陷阱”驱动的抗CD47 siRNA抑制肺癌细胞的生长
使用当前基于EPR效应和配体-受体相互作用的肿瘤选择性药物递送方法,效率还不够高,并且它们在很大程度上未能转化为临床。因此,迫切需要探索一种有效递送抗癌药的增强策略。临床上,许多癌症需要大量的谷氨酰胺以使其持续生长和存活,从而导致肿瘤中循环谷氨酰胺的提取量大大超过任何器官,表现为“谷氨酰胺陷阱”。在本研究中,我们试图阐明是否可以利用谷氨酰胺捕获作用来递送治疗剂以选择性杀死癌细胞。这里是大分子谷氨酰胺类似物,谷氨酰胺官能化的支链聚乙烯亚胺(GPI),被构建为运送抗CD47 siRNA的载体,以阻断癌细胞上CD47“不要吃我”的信号。GPI / siRNA富含谷氨酰胺的多聚体对依赖谷氨酰胺的肺癌细胞,野生型A549细胞(A549WT)及其顺铂耐药细胞(A549 DDP),特别是在谷氨酰胺耗尽的条件下。注意到谷氨酰胺转运蛋白ASCT2在A549 WT和A549 DDP上均高表达但在正常人肺成纤维细胞中几乎没有表达。ASCT2的抑制作用显着阻止了GPI多聚体的内在化。这些发现提出了有趣的可能性,即富含谷氨酰胺的GPI多聚体利用ASCT2途径选择性地促进癌细胞对它们的细胞摄取。GPI进一步在体内和体外有效递送抗CD47 siRNA,以下调CD47的肿瘤内mRNA和蛋白质表达水平。CD47充当“不要吃我”的信号,并与免疫受体SIRPα结合,导致逃避吞噬作用清除。GPI /抗CD47 siRNA复合物在A549 WT和A549 DDP上均实现了显着的抗肿瘤活性荷瘤裸鼠。值得注意的是,它对表达CD47的红细胞和血小板没有不利影响,可能是由于选择性递送所致。因此,由谷氨酰胺捕获效应驱动的富含谷氨酰胺的载体GPI为设计抗癌药物递送系统提供了有希望的新策略。
更新日期:2018-06-25
中文翻译:

富含谷氨酰胺的载体可有效提供“谷氨酰胺陷阱”驱动的抗CD47 siRNA抑制肺癌细胞的生长
使用当前基于EPR效应和配体-受体相互作用的肿瘤选择性药物递送方法,效率还不够高,并且它们在很大程度上未能转化为临床。因此,迫切需要探索一种有效递送抗癌药的增强策略。临床上,许多癌症需要大量的谷氨酰胺以使其持续生长和存活,从而导致肿瘤中循环谷氨酰胺的提取量大大超过任何器官,表现为“谷氨酰胺陷阱”。在本研究中,我们试图阐明是否可以利用谷氨酰胺捕获作用来递送治疗剂以选择性杀死癌细胞。这里是大分子谷氨酰胺类似物,谷氨酰胺官能化的支链聚乙烯亚胺(GPI),被构建为运送抗CD47 siRNA的载体,以阻断癌细胞上CD47“不要吃我”的信号。GPI / siRNA富含谷氨酰胺的多聚体对依赖谷氨酰胺的肺癌细胞,野生型A549细胞(A549WT)及其顺铂耐药细胞(A549 DDP),特别是在谷氨酰胺耗尽的条件下。注意到谷氨酰胺转运蛋白ASCT2在A549 WT和A549 DDP上均高表达但在正常人肺成纤维细胞中几乎没有表达。ASCT2的抑制作用显着阻止了GPI多聚体的内在化。这些发现提出了有趣的可能性,即富含谷氨酰胺的GPI多聚体利用ASCT2途径选择性地促进癌细胞对它们的细胞摄取。GPI进一步在体内和体外有效递送抗CD47 siRNA,以下调CD47的肿瘤内mRNA和蛋白质表达水平。CD47充当“不要吃我”的信号,并与免疫受体SIRPα结合,导致逃避吞噬作用清除。GPI /抗CD47 siRNA复合物在A549 WT和A549 DDP上均实现了显着的抗肿瘤活性荷瘤裸鼠。值得注意的是,它对表达CD47的红细胞和血小板没有不利影响,可能是由于选择性递送所致。因此,由谷氨酰胺捕获效应驱动的富含谷氨酰胺的载体GPI为设计抗癌药物递送系统提供了有希望的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号