当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Recent development of ortho-C–H functionalization of aryl sulfoxides through [3,3] sigmatropic rearrangement
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-06-26 , DOI: 10.1016/j.tetlet.2018.06.055 Tomoyuki Yanagi , Keisuke Nogi , Hideki Yorimitsu
中文翻译:

通过[3,3]σ重排的芳基亚砜邻-C–H官能化的最新进展
更新日期:2018-06-26
Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-06-26 , DOI: 10.1016/j.tetlet.2018.06.055 Tomoyuki Yanagi , Keisuke Nogi , Hideki Yorimitsu
|
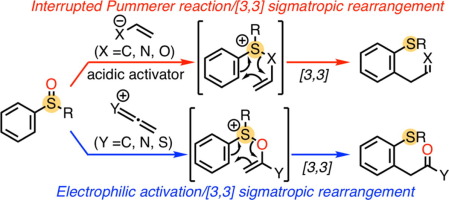
Sulfoxide-promoted ortho-C–H functionalization reactions of aromatic compounds have emerged as powerful tools for organic synthesis. Among them, [3,3] sigmatropic rearrangement induced by interrupted Pummerer reaction or electrophilic activation of sulfoxides has proved to be a fruitful strategy due to its versatility and high reaction efficiency. This digest paper mainly focuses on recent progress on C–H functionalization reactions of aryl sulfoxides via [3,3] sigmatropic rearrangement of sulfonium-tethered intermediates.
中文翻译:

通过[3,3]σ重排的芳基亚砜邻-C–H官能化的最新进展
亚砜促进的芳族化合物邻位-C-H官能化反应已成为有机合成的有力工具。其中,由于中断的Pummerer反应或亚砜的亲电活化引起的[3,3]σ重排已被证明是一项富有成效的策略,因为它具有多功能性和高反应效率。本摘要文章主要关注芳基亚砜通过[3,3] igma系链中间体的σ重排的C–H官能化反应的最新进展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号