Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of arrays of planar, murine, intestinal crypts possessing a stem/proliferative cell compartment and differentiated cell zone†
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1039/c8lc00332g Raehyun Kim 1 , Yuli Wang , Shee-Hwan J Hwang , Peter J Attayek , Nicole M Smiddy , Mark I Reed , Christopher E Sims , Nancy L Allbritton
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-06-26 00:00:00 , DOI: 10.1039/c8lc00332g Raehyun Kim 1 , Yuli Wang , Shee-Hwan J Hwang , Peter J Attayek , Nicole M Smiddy , Mark I Reed , Christopher E Sims , Nancy L Allbritton
Affiliation
|
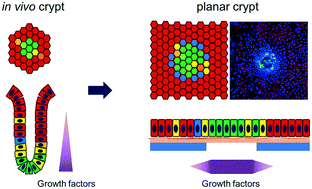
A simple, in vitro intestinal model recapitulating key aspects of crypt architecture and physiology would facilitate our understanding the impact of drugs, foods and microbial metabolites on the intestine. To address the limitations of previously reported intestinal in vitro platforms, we developed a planar crypt array that replicated the spatial segregation and physiologic responses of primary mouse intestinal epithelial cells in the large intestine. Collagen was coated across an impermeable film possessing an array of microholes creating two regions of distinct stiffness and porosity (above and outside the microholes). Primary mouse colon epithelial cells formed a continuous monolayer across the array with a proliferative cell zone above the microholes and a nonproliferative or differentiated cell region distant from the microholes. Formation of a chemical gradient of growth factors across the array yielded a more complete or in vivo-like cell segregation of proliferative and differentiated cells with cell migration outward from the proliferative cell zone into the differentiated zone to replace apoptotic dying cells much as occurs in vivo. Short chain fatty acids (microbial metabolites) applied to the luminal surface of the crypt array significantly impacted the proliferation and differentiation of the cells replicating the known in vivo effects of these fatty acids. Importantly this planar crypt array was readily fabricated and maintained, easily imaged with properties quantified by microscopy, and compatible with reagent addition to either the luminal or basal fluid reservoirs. The ability to observe simultaneously stem/proliferative and differentiated cell behavior and movement between these two compartments in response to drugs, toxins, inflammatory mediators or microbial metabolites will be of widespread utility.
中文翻译:

形成具有干/增殖细胞区室和分化细胞区的平面鼠类肠隐窝阵列†
一个简单的体外肠道模型概括了隐窝结构和生理学的关键方面,将有助于我们了解药物、食物和微生物代谢物对肠道的影响。为了解决先前报道的肠道体外平台的局限性,我们开发了一种平面隐窝阵列,该阵列复制了大肠中原代小鼠肠上皮细胞的空间分离和生理反应。将胶原蛋白涂在具有一系列微孔的不渗透薄膜上,形成两个具有不同硬度和孔隙率的区域(微孔上方和外部)。原代小鼠结肠上皮细胞在阵列上形成连续的单层,其中在微孔上方具有增殖细胞区域,以及远离微孔的非增殖或分化细胞区域。整个阵列中生长因子化学梯度的形成产生了增殖和分化细胞的更完整或类似体内的细胞分离,细胞从增殖细胞区域向外迁移到分化区域以取代凋亡的垂死细胞,就像体内发生的那样。应用于隐窝阵列管腔表面的短链脂肪酸(微生物代谢物)显着影响细胞的增殖和分化,复制这些脂肪酸的已知体内效应。重要的是,这种平面隐窝阵列易于制造和维护,易于通过显微镜量化的特性进行成像,并且与向管腔或基础流体储库中添加试剂兼容。 同时观察干/增殖和分化细胞行为以及这两个区室之间响应药物、毒素、炎症介质或微生物代谢物的运动的能力将具有广泛的用途。
更新日期:2018-06-26
中文翻译:

形成具有干/增殖细胞区室和分化细胞区的平面鼠类肠隐窝阵列†
一个简单的体外肠道模型概括了隐窝结构和生理学的关键方面,将有助于我们了解药物、食物和微生物代谢物对肠道的影响。为了解决先前报道的肠道体外平台的局限性,我们开发了一种平面隐窝阵列,该阵列复制了大肠中原代小鼠肠上皮细胞的空间分离和生理反应。将胶原蛋白涂在具有一系列微孔的不渗透薄膜上,形成两个具有不同硬度和孔隙率的区域(微孔上方和外部)。原代小鼠结肠上皮细胞在阵列上形成连续的单层,其中在微孔上方具有增殖细胞区域,以及远离微孔的非增殖或分化细胞区域。整个阵列中生长因子化学梯度的形成产生了增殖和分化细胞的更完整或类似体内的细胞分离,细胞从增殖细胞区域向外迁移到分化区域以取代凋亡的垂死细胞,就像体内发生的那样。应用于隐窝阵列管腔表面的短链脂肪酸(微生物代谢物)显着影响细胞的增殖和分化,复制这些脂肪酸的已知体内效应。重要的是,这种平面隐窝阵列易于制造和维护,易于通过显微镜量化的特性进行成像,并且与向管腔或基础流体储库中添加试剂兼容。 同时观察干/增殖和分化细胞行为以及这两个区室之间响应药物、毒素、炎症介质或微生物代谢物的运动的能力将具有广泛的用途。











































 京公网安备 11010802027423号
京公网安备 11010802027423号