当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergetic effect of H and He impurities in Ti3AlC2: first principles calculations
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8cp02082e Jiajia Liu 1, 2, 3, 4 , Canglong Wang 3, 4, 5, 6 , Xiaolu Zhu 1, 2, 3, 4 , Jitao Liu 3, 4, 5, 6 , Xingming Zhang 4, 7, 8, 9 , Xueqiang Gou 1, 2, 3, 4 , Wenshan Duan 1, 2, 3, 4 , Lei Yang 3, 4, 5, 6
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8cp02082e Jiajia Liu 1, 2, 3, 4 , Canglong Wang 3, 4, 5, 6 , Xiaolu Zhu 1, 2, 3, 4 , Jitao Liu 3, 4, 5, 6 , Xingming Zhang 4, 7, 8, 9 , Xueqiang Gou 1, 2, 3, 4 , Wenshan Duan 1, 2, 3, 4 , Lei Yang 3, 4, 5, 6
Affiliation
|
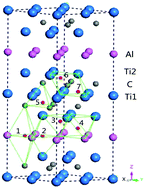
First principles calculations have been performed to investigate the synergetic effect of H and He impurities with vacancies in Ti3AlC2. The configurations and energetics of Hn–He–VAl complexes (n ≤ 4) and He–He/He–H/H–H interactions have been studied. It is found that the impurity H atom prefers to occupy the tetrahedral interstitial site (Itetr-3), but the He atom prefers to occupy the octahedral interstitial site (Ioct-4) in perfect Ti3AlC2. Within a pre-existing Al vacancy, the most favorable site for a He atom is close to tetr-site, meanwhile the H atom preferentially deviates from the vacancy center with the separation 1.3 Å along the 〈001〉 direction. He–H and He–He show a weakly attractive interaction, but weak repulsion occurs in the H–H interaction, which is different from the case of Ti3SiC2. The He–VAl complex plays an important role in the trapping of H atoms. The He–VAl cluster can trap up to three H atoms in the absence of H2 molecules, which leads to the formation of a H–He hybridized bubble. Thus, the He atom can subsequently suppress further aggregation of H atoms and block hydrogen embrittlement and volume swelling.
中文翻译:

H 3和He杂质在Ti 3 AlC 2中的协同作用:第一性原理计算
已经进行了第一原理计算,以研究H和He杂质与Ti 3 AlC 2中的空位的协同作用。的配置和H的能量学ñ -他-V的Al配合物(Ñ ≤4)和He-他/他-H / H-H的相互作用进行了研究。发现杂质H原子更倾向于占据四面体间隙位(I tetr -3),而He原子更倾向于占据完美Ti 3 AlC 2中的八面体间隙位(I oct -4)。。在预先存在的Al空位中,He原子最有利的位置靠近四原子位,同时H原子优先偏离空位中心,沿<001>方向的间距为1.3Å。He–H和He–He表现出弱的吸引力相互作用,但在H–H相互作用中产生弱排斥力,这与Ti 3 SiC 2的情况不同。He-V Al络合物在俘获H原子中起重要作用。在没有H 2分子的情况下,He–V Al团簇最多可以俘获3个H原子,这导致形成H–He杂化气泡。因此,He原子可随后抑制H原子的进一步聚集,并阻止氢脆和体积膨胀。
更新日期:2018-06-25
中文翻译:

H 3和He杂质在Ti 3 AlC 2中的协同作用:第一性原理计算
已经进行了第一原理计算,以研究H和He杂质与Ti 3 AlC 2中的空位的协同作用。的配置和H的能量学ñ -他-V的Al配合物(Ñ ≤4)和He-他/他-H / H-H的相互作用进行了研究。发现杂质H原子更倾向于占据四面体间隙位(I tetr -3),而He原子更倾向于占据完美Ti 3 AlC 2中的八面体间隙位(I oct -4)。。在预先存在的Al空位中,He原子最有利的位置靠近四原子位,同时H原子优先偏离空位中心,沿<001>方向的间距为1.3Å。He–H和He–He表现出弱的吸引力相互作用,但在H–H相互作用中产生弱排斥力,这与Ti 3 SiC 2的情况不同。He-V Al络合物在俘获H原子中起重要作用。在没有H 2分子的情况下,He–V Al团簇最多可以俘获3个H原子,这导致形成H–He杂化气泡。因此,He原子可随后抑制H原子的进一步聚集,并阻止氢脆和体积膨胀。










































 京公网安备 11010802027423号
京公网安备 11010802027423号