当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly diastereoselective synthesis of tricyclic fused-pyrans by sequential hydride shift mediated double C(sp3)–H bond functionalization†
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8sc02103a Keiji Mori 1, 2 , Nobuaki Umehara 1 , Takahiko Akiyama 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-06-25 00:00:00 , DOI: 10.1039/c8sc02103a Keiji Mori 1, 2 , Nobuaki Umehara 1 , Takahiko Akiyama 1
Affiliation
|
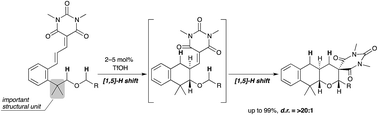
Described herein is the Brønsted acid-catalyzed double C(sp3)–H bond functionalization of alkyl phenethyl ether derivatives. In this process, a [1,5]-[1,5]-hydride shift occurred successively to afford tricyclic fused pyran derivatives in excellent chemical yields with excellent diastereoselectivities (up to >20 : 1). The key to achieve this reaction was the introduction of two methyl groups at the benzylic position, which was effective in both hydride shift processes: (1) the Thorpe–Ingold effect for the first hydride shift and (2) conformational control in the second hydride shift.
中文翻译:

通过连续氢化物位移介导的双 C(sp3)–H 键功能化高度非对映选择性合成三环稠合吡喃†
本文描述的是布朗斯台德酸催化的烷基苯乙基醚衍生物的双C(sp 3 )-H键官能化。在此过程中,[1,5]-[1,5]-氢化物连续发生转变,以优异的化学收率和优异的非对映选择性(高达%3E20:1)提供三环稠合吡喃衍生物。实现该反应的关键是在苄基位置引入两个甲基,这在两个氢化物转移过程中都有效:(1)第一次氢化物转移的索普-英戈尔德效应和(2)第二次氢化物中的构象控制转移。
更新日期:2018-06-25
中文翻译:

通过连续氢化物位移介导的双 C(sp3)–H 键功能化高度非对映选择性合成三环稠合吡喃†
本文描述的是布朗斯台德酸催化的烷基苯乙基醚衍生物的双C(sp 3 )-H键官能化。在此过程中,[1,5]-[1,5]-氢化物连续发生转变,以优异的化学收率和优异的非对映选择性(高达%3E20:1)提供三环稠合吡喃衍生物。实现该反应的关键是在苄基位置引入两个甲基,这在两个氢化物转移过程中都有效:(1)第一次氢化物转移的索普-英戈尔德效应和(2)第二次氢化物中的构象控制转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号