当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Boscalid via a three-step telescoped continuous flow process implemented on a MJOD reactor platform†
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-06-23 00:00:00 , DOI: 10.1039/c8re00049b Audun Drageset 1, 2, 3, 4 , Vijayaragavan Elumalai 1, 2, 3, 4 , Hans-René Bjørsvik 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-06-23 00:00:00 , DOI: 10.1039/c8re00049b Audun Drageset 1, 2, 3, 4 , Vijayaragavan Elumalai 1, 2, 3, 4 , Hans-René Bjørsvik 1, 2, 3, 4
Affiliation
|
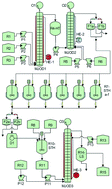
A three step continuous/semi-flow process leading to the fungicide Boscalid® is disclosed. The first step of the process is a Suzuki cross-coupling where 1-chloro-2-nitrobenzene is coupled with 4-chlorophenylboronic acid at a temperature of 80 °C using a solvent mixture of ethanol/water as the reaction medium with sodium carbonate as the base and tetrakis(triphenylphosphine)-palladium as the catalyst. This reaction step was developed as a batch procedure and then adapted and implemented on a multi-jet oscillating disk (MJOD) continuous flow reactor platform. The intermediate 4′-chloro-2-nitro-1,1′-biphenyl was achieved in high yield (82%). The second step which was telescoped with the first one involved a nitro group reduction of high efficacy. The reduction method was based on NaBH4/CoSO4·7H2O as the reduction system. The reduction product 2-amino-4′-chloro-1,1′-biphenyl was achieved in high yield (79%) at a short reactor residence time (3 min). The final step of the process is composed of a two-step sequence where 2-amino-4′-chloro-1,1′-biphenyl is transformed into the corresponding iminosulfanone intermediate (not isolated), which immediately reacts with the 2-chloronicotinic acid present in the reaction mixture to form Boscalid® in a yield of >66%. The three-step process provided an overall yield of >42%, which corresponds to a mean step yield of ≈75%.
中文翻译:

通过在MJOD反应器平台上实施的三步伸缩连续流法 合成Boscalid †
公开了导致杀真菌剂的三步连续/半流过程。该方法的第一步是铃木交叉偶联,其中将1-氯-2-硝基苯与4-氯苯基硼酸在80°C的温度下偶联,使用乙醇/水的溶剂混合物作为反应介质,并用碳酸钠作为反应介质。碱和四(三苯基膦)-钯作为催化剂。该反应步骤是作为分批程序开发的,然后在多喷嘴振荡盘(MJOD)连续流反应器平台上进行调整和实施。以高收率(82%)获得中间体4'-氯-2-硝基-1,1'-联苯。用第一步进行伸缩的第二步涉及高效还原硝基。还原方法基于NaBH 4 / CoSO 4·7H 2 O作为还原系统。在短的反应器停留时间(3分钟)下以高收率(79%)获得了还原产物2-氨基-4′-氯-1,1′-联苯。该方法的最后一步由两步组成,其中将2-氨基-4'-氯-1,1'-联苯转化为相应的亚氨基砜中间体(未分离),该中间体立即与2-氯烟碱反应存在于反应混合物中的丙烯酸形成Boscalid®,收率> 66%。三步过程提供了> 42%的总产率,相当于≈75%的平均分步产率。
更新日期:2018-06-23
中文翻译:

通过在MJOD反应器平台上实施的三步伸缩连续流法 合成Boscalid †
公开了导致杀真菌剂的三步连续/半流过程。该方法的第一步是铃木交叉偶联,其中将1-氯-2-硝基苯与4-氯苯基硼酸在80°C的温度下偶联,使用乙醇/水的溶剂混合物作为反应介质,并用碳酸钠作为反应介质。碱和四(三苯基膦)-钯作为催化剂。该反应步骤是作为分批程序开发的,然后在多喷嘴振荡盘(MJOD)连续流反应器平台上进行调整和实施。以高收率(82%)获得中间体4'-氯-2-硝基-1,1'-联苯。用第一步进行伸缩的第二步涉及高效还原硝基。还原方法基于NaBH 4 / CoSO 4·7H 2 O作为还原系统。在短的反应器停留时间(3分钟)下以高收率(79%)获得了还原产物2-氨基-4′-氯-1,1′-联苯。该方法的最后一步由两步组成,其中将2-氨基-4'-氯-1,1'-联苯转化为相应的亚氨基砜中间体(未分离),该中间体立即与2-氯烟碱反应存在于反应混合物中的丙烯酸形成Boscalid®,收率> 66%。三步过程提供了> 42%的总产率,相当于≈75%的平均分步产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号