Cellular Signalling ( IF 4.4 ) Pub Date : 2018-06-23 , DOI: 10.1016/j.cellsig.2018.06.010 Lizzy Wanka , Stefanie Babilon , Anette Kaiser , Karin Mörl , Annette G. Beck-Sickinger
|
GPCR internalization, which is induced by arrestin recruitment, is an important mechanism for the regulation of signaling and receptor quantity at the cell surface.
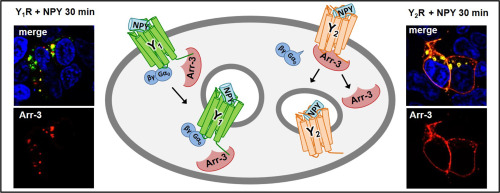
In this study, differences in the mechanism of arrestin-3 (arr-3) recruitment to the neuropeptide Y1 and Y2 receptor were identified. These receptors play an essential role in the regulation of feeding, energy homeostasis and cancer. The Y1R displays high affinity to arr-3, which induces rapid internalization of the arrestin/receptor complex. In contrast, the Y2R has a lower affinity for arr-3. Internalization is induced by arrestin binding, but arr-3 is released from the receptor and remains at the membrane while the receptor internalizes. Moreover, the deletion of the finger loop region of arr-3 reduces its agonist-dependent recruitment to the Y2R significantly, but not to the Y1R suggesting different binding conformations.
For the first time, the formation of a supercomplex consisting of Y receptor, Gα0 protein and arrestin was studied by BRET-assay. We demonstrated that the Y1R is able to bind Gα0 protein as well as arr-3 simultaneously and internalizes as a supercomplex. For the Y2R no supercomplex formation was observed.
By substituting the C-terminus or specific residues within the intracellular loop 1 and 2 of the receptors, the arr-3 recruitment of the Y1R and Y2R can be switched. Thus, we shed light on the specific spatio-temporal distribution of Gα0 protein and arrestin in response to Y1 versus Y2 receptor activation and identified the molecular determinants.
中文翻译:

人Y 1和Y 2受体上restarin-3结合的不同模式
由抑制蛋白募集诱导的GPCR内在化是调节细胞表面信号和受体数量的重要机制。
在这项研究中,发现了抑制素3(arr-3)募集到神经肽Y 1和Y 2受体的机制上的差异。这些受体在调节喂养,能量稳态和癌症中起着至关重要的作用。Y 1 R对arr-3表现出高亲和力,从而诱导抑制蛋白/受体复合物快速内在化。相反,Y 2 R对arr-3的亲和力较低。内部抑制是通过抑制素结合而诱导的,但是arr-3会从受体释放,并在受体内部化时保留在膜上。此外,arr-3的手指环区域的删除大大减少了其激动剂依赖性募集到Y 2 R,而不是Y 1 RR暗示不同的结合构象。
首次,由Y受体的超复合的形成,Gα 0蛋白质和抑制蛋白通过BRET的测定研究。我们表明,在Y 1,R是能够结合Gα 0蛋白质以及ARR-3同时且内化作为超复合。对于Y 2 R,未观察到超复合物的形成。
通过取代受体的细胞内环1和2内的C端或特定残基,可以切换Y 1 R和Y 2 R的arr-3募集。因此,我们阐明了Gα的特定时空分布0响应于Y蛋白和抑制蛋白1与ý 2受体激活和识别的分子决定因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号