Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-06-23 , DOI: 10.1016/j.bmc.2018.06.030 Yazmín Arellano , Eugene Bratoeff , Yvonne Heuze , Marisol Bravo , Juan Soriano , Marisa Cabeza
|
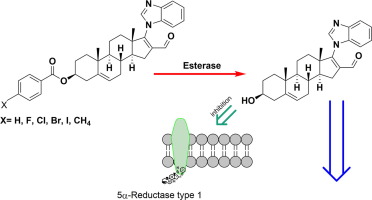
It is known that the growth of prostate metastatic bone tumor depends on androgens, and tumor formation can start from migratory malignant cells produced in that organ. These cells exhibit grater type 1 5α-reductase (5α-R1) activity than type 2 5α-reductase. Noteworthy, both isozymes convert testosterone (T) to the more active androgen dihydrotestosterone (DHT) in the target tissues.
Thus, in order to potentially improve the prognosis of this disease, in this work, seven derivatives of 17-(1H-benzimidazol-1-yl)-16-formillandrosta-5,16-dien-3β-yl benzoate (4a–f) and 17-(1H-benzimidazol-1-yl)-3-hydroxy-16-formylandrost-5,16-diene (4) were synthesized, characterized and identified as inhibitors of type 1 5α-reductase (5αR1). These derivatives having the advantage of improved plasma half-life.
The inhibitory activity of the compounds towards 5α-R1 isoenzyme was determined by conversion of T into DHT in the presence or absence of compounds 4, 4a–f. Further, in vivo experiments were also carried out, treating gonadectomized hamsters with T and/or 4, 4a–f and evaluating their effect on the diameter of hamster flank organs and on the weight of the prostatic and seminal vesicles. Results indicated that compounds 4, 4b, 4c, served as in vitro inhibitors of the enzyme 5α-R1 and pharmacological experiments showed that 4 and derivatives 4a–f decreased the diameter of the flank glands, the weight of the prostate and seminal vesicles of treated hamsters without any appreciable toxicity during observation. Noteworthy the fact that compound 4 is the product, in all cases, of the hydrolysis of the series of esters 4a–f, thus they can serve as precursors (prodrugs) of the active form 4.
中文翻译:

类固醇4及其衍生物4a–4f作为5α-还原酶1抑制剂的活性
已知前列腺转移性骨肿瘤的生长取决于雄激素,并且肿瘤形成可以从该器官中产生的迁移性恶性细胞开始。这些细胞比2型5α还原酶具有1型5α还原酶(5α-R1)活性。值得注意的是,两种同工酶都在靶组织中将睾丸激素(T)转化为活性更高的雄激素二氢睾丸激素(DHT)。
因此,为了潜在地改善该疾病的预后,在这项工作中,使用了17-(1 H-苯并咪唑-1-基)-16-甲腈-5,16-二烯-3β-苯甲酸酯的7种衍生物(4a–合成了f)和17-(1 H-苯并咪唑-1-基)-3-羟基-16-甲酰二茂基-5,16-二烯(4),并将其鉴定为1型5α-还原酶(5αR1)的抑制剂。这些衍生物具有改善的血浆半衰期的优点。
向5α-R1同工酶的化合物的抑制活性是通过转化的T到DHT中的存在或不存在化合物的确定4,图4a-f的。此外,在体内实验也在进行,治疗与T和/或去势仓鼠4,图4a-f的和仓鼠的直径评价其效果侧翼器官和在前列腺和精囊的重量。结果表明,化合物4,图4b,图4c,担任体外酶5α-R1和药理实验的抑制剂表明,4和衍生物4A-F减少了处理过的仓鼠的胁腹直径,前列腺和精囊的重量,在观察过程中没有任何明显的毒性。值得注意的事实是,化合物4在所有情况下都是一系列酯4a-f水解的产物,因此它们可以用作活性形式4的前体(前药)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号