当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 1,4-Thiazepines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-24 00:00:00 , DOI: 10.1021/acs.joc.8b01029 Yilmaz Kelgokmen 1 , Metin Zora 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-24 00:00:00 , DOI: 10.1021/acs.joc.8b01029 Yilmaz Kelgokmen 1 , Metin Zora 1
Affiliation
|
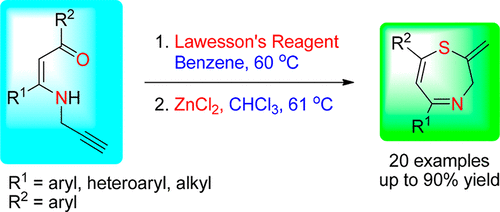
An efficient, general, and unprecedented methodology for the synthesis of 2-methylene-2,3-dihydro-1,4-thiazepines from N-propargylic β-enaminones is described. Initially, N-propargylic β-enaminones were thionated with Lawesson’s reagent in good to high yields, and then the resulting N-propargylic β-enaminothiones were subjected to electrophilic cyclization. When treated with zinc chloride in refluxing chloroform, N-propargylic β-enaminothiones underwent electrophilic cyclization to yield 2-methylene-2,3-dihydro-1,4-thiazepines in good to high yields. A general trend was observed for all N-propargylic β-enaminothiones, and the cyclization proceeded with high efficiency and large functional group tolerance. This process is also applicable to the cyclization of internal alkyne-tethered N-propargylic β-enaminothiones. This operationally simple and facile method may represent a very rapid entry to a library of functionalized 1,4-thiazepines in the area of pharmaceuticals.
中文翻译:

1,4-噻嗪类的合成
描述了一种由N-炔丙基β-烯胺酮合成2-亚甲基-2,3-二氢-1,4-噻氮平的有效,通用且空前的方法。最初,将N-炔丙基β-烯胺酮用Lawesson试剂以高产率至高产率进行亚硫酰化,然后将所得的N-炔丙基β-烯胺酮进行亲电环化。当在回流的氯仿中用氯化锌处理时,对N-炔丙基β-烯氨基硫酮进行亲电子环化,以良好或高收率得到2-亚甲基-2,3-二氢-1,4-噻嗪类。观察到所有N的总体趋势-炔丙基β-烯胺硫酮,并且环化以高效率和大官能团耐受性进行。该方法也适用于内部炔烃连接的N-炔丙基β-烯胺硫酮的环化。这种操作简单,简便的方法可以代表非常快速地进入制药领域中功能化的1,4-硫氮平的文库。
更新日期:2018-06-24
中文翻译:

1,4-噻嗪类的合成
描述了一种由N-炔丙基β-烯胺酮合成2-亚甲基-2,3-二氢-1,4-噻氮平的有效,通用且空前的方法。最初,将N-炔丙基β-烯胺酮用Lawesson试剂以高产率至高产率进行亚硫酰化,然后将所得的N-炔丙基β-烯胺酮进行亲电环化。当在回流的氯仿中用氯化锌处理时,对N-炔丙基β-烯氨基硫酮进行亲电子环化,以良好或高收率得到2-亚甲基-2,3-二氢-1,4-噻嗪类。观察到所有N的总体趋势-炔丙基β-烯胺硫酮,并且环化以高效率和大官能团耐受性进行。该方法也适用于内部炔烃连接的N-炔丙基β-烯胺硫酮的环化。这种操作简单,简便的方法可以代表非常快速地进入制药领域中功能化的1,4-硫氮平的文库。











































 京公网安备 11010802027423号
京公网安备 11010802027423号