Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformations and Dynamic Transitions of a Melittin Derivative That Forms Macromolecule-Sized Pores in Lipid Bilayers
Langmuir ( IF 3.7 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.langmuir.8b00804 Anna E. Pittman , Brendan P. Marsh , Gavin M. King
Langmuir ( IF 3.7 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.langmuir.8b00804 Anna E. Pittman , Brendan P. Marsh , Gavin M. King
|
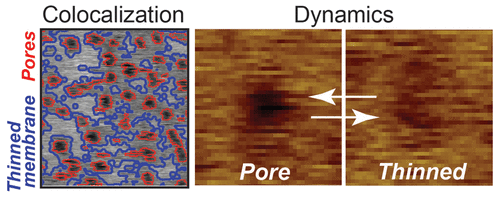
Systematically evolved from the primary active component of bee venom, MelP5 is a lipophilic peptide with important physical properties that differ from wild-type melittin, including the ability to create large equilibrium pores in lipid bilayers at low peptide to lipid ratios. Self-assembly into stable membrane spanning pores makes MelP5 a promising candidate for future applications in the pharmaceutical arena. Despite significant interest, little is known about the mechanism by which MelP5 remodels the lipid bilayer upon binding. We demonstrate by direct atomic force microscope imaging of supported lipid bilayers in solution that MelP5 remodels 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) in one of two ways. It creates either highly localized voids in the bilayer or diffuse nonlocalized thinning. Thinning of the bilayer was measured to be 3.0 ± 1.4 Å (mean ± standard deviation) below the surface of the upper leaflet of the bilayer. Pores, defined as highly localized voids in the bilayer, exhibited several sizes. Approximately 20% of pores exhibited large footprint areas (47 ± 20 nm2) which appear capable of passing bulky macromolecules. The peptide-effected bilayer was observed to reversibly exchange between membrane-thinned and pore states in an apparent dynamic equilibrium. Analysis of time-lapsed images suggested upper and lower bounds (0.2 < τ < 180 s) on the characteristic time scale of transitions between the membrane-thinned and pore states. Moreover, pores were found to colocalize with membrane-thinned regions, a novel observation that is consistent with the notion of cooperativity among membrane-bound peptides when forming pores.
中文翻译:

蜂胶衍生物在脂质双层中形成大分子孔的构象和动态转变。
MelP5是从蜂毒的主要活性成分系统地进化而来的,是一种亲脂性肽,具有与野生型蜂毒肽不同的重要物理特性,包括以低肽与脂质比率在脂质双层中形成大的平衡孔的能力。自组装成稳定的跨膜孔使MelP5成为未来在制药领域的应用的有希望的候选者。尽管引起了极大的兴趣,但关于MelP5结合后重塑脂质双层的机制知之甚少。我们通过直接原子力显微镜对溶液中支持的脂质双层的成像进行了演示,表明MelP5重塑了1-palmitoyl-2-oleoyl- sn-甘油-3-磷酸胆碱(POPC)的两种方法之一。它在双层中产生高度局部化的空隙或扩散非局部性变薄。双层的变薄测得为双层的上小叶表面以下3.0±1.4Å(平均±标准偏差)。毛孔,定义为双层中高度局部化的空隙,表现出几种尺寸。大约20%的孔显示出较大的覆盖面积(47±20 nm 2)似乎能够通过大分子。观察到肽作用的双层在明显的动态平衡中在膜变薄的和孔状态之间可逆地交换。对延时图像的分析表明,在膜变薄和孔隙状态之间的过渡特征时间尺度上,存在上限和下限(0.2 <τ<180 s)。此外,发现孔与膜变薄的区域共定位,这是一种新颖的观察结果,与形成孔时膜结合肽之间的协同作用概念一致。
更新日期:2018-06-22
中文翻译:

蜂胶衍生物在脂质双层中形成大分子孔的构象和动态转变。
MelP5是从蜂毒的主要活性成分系统地进化而来的,是一种亲脂性肽,具有与野生型蜂毒肽不同的重要物理特性,包括以低肽与脂质比率在脂质双层中形成大的平衡孔的能力。自组装成稳定的跨膜孔使MelP5成为未来在制药领域的应用的有希望的候选者。尽管引起了极大的兴趣,但关于MelP5结合后重塑脂质双层的机制知之甚少。我们通过直接原子力显微镜对溶液中支持的脂质双层的成像进行了演示,表明MelP5重塑了1-palmitoyl-2-oleoyl- sn-甘油-3-磷酸胆碱(POPC)的两种方法之一。它在双层中产生高度局部化的空隙或扩散非局部性变薄。双层的变薄测得为双层的上小叶表面以下3.0±1.4Å(平均±标准偏差)。毛孔,定义为双层中高度局部化的空隙,表现出几种尺寸。大约20%的孔显示出较大的覆盖面积(47±20 nm 2)似乎能够通过大分子。观察到肽作用的双层在明显的动态平衡中在膜变薄的和孔状态之间可逆地交换。对延时图像的分析表明,在膜变薄和孔隙状态之间的过渡特征时间尺度上,存在上限和下限(0.2 <τ<180 s)。此外,发现孔与膜变薄的区域共定位,这是一种新颖的观察结果,与形成孔时膜结合肽之间的协同作用概念一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号