Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ionic Strength-Responsive Binding between Nanoparticles and Proteins
Langmuir ( IF 3.7 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.langmuir.8b00944 Xiaohan Wang 1 , Shi Zhang 1 , Yisheng Xu 1, 2, 3 , Xiaotao Zhao 1 , Xuhong Guo 1, 2
Langmuir ( IF 3.7 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.langmuir.8b00944 Xiaohan Wang 1 , Shi Zhang 1 , Yisheng Xu 1, 2, 3 , Xiaotao Zhao 1 , Xuhong Guo 1, 2
Affiliation
|
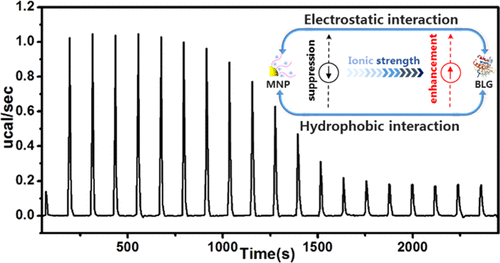
Electrostatic interaction is a strong, dominant nonspecific interaction which was extensively studied in protein–nanoparticle (NP) interactions [Lounis, F. M. ; J. Phys. Chem. B 2017, 121, 2684−2694; Tavares, G. M. ; Langmuir 2015, 31, 12481–12488; Antonov, M. ; Biomacromolecules 2010, 11, 51–59], whereas the role of hydrophobic interaction arising from the abundant hydrophobic residues of globule proteins upon protein–NP binding between the proteins and charged nanoparticles has rarely been studied. In this work, a series of positively charged magnetic nanoparticles (MNPs) were prepared via atom transfer radical polymerization and surface hydrophobicity differentiation was achieved through postpolymerization quaternization by different halohydrocarbons. The ionic strength- and hydrophobicity-responsive binding of these MNPs toward β-lactoglobulin (BLG) was studied by both qualitative and quantitative methods including turbidimetric titration, dynamic light scattering, and isothermal titration calorimetry. Judged from the critical binding pH and binding constant for MNP–BLG complexation, the dependence of binding affinity on surface hydrophobicity exhibited an interesting shift with increasing ionic strength, which means that the MNPs with higher surface hydrophobicity exhibits weaker binding affinity at lower ionic strength but stronger affinity at higher ionic strength. This interesting observation could be attributed to the difference in ionic strength responsiveness for hydrophobic and electrostatic interactions. In this way, the well-tuned binding pattern could be achieved with optimized binding affinity by controlling the surface hydrophobicity of MNPs and ionic strength, thus endowing this system with great potential to fabricate separation and delivery system with high selectivity and efficiency.
中文翻译:

离子强度响应纳米粒子和蛋白质之间的绑定。
静电相互作用是一种强大的,占优势的非特异性相互作用,已在蛋白质-纳米颗粒(NP)相互作用中进行了广泛研究[FM路易斯尼斯 ; J.物理 化学 乙 2017,121,2684年至2694年;塔瓦雷斯,通用 ; 朗缪尔 2015,31,12481-12488;安东诺夫(美国) ; 生物大分子 2010,11(51-59),而由球形蛋白的大量疏水残基引起的疏水相互作用对蛋白质与带电纳米粒子之间的蛋白质-NP结合的作用却鲜有研究。在这项工作中,通过原子转移自由基聚合制备了一系列带正电的磁性纳米粒子(MNP),并通过不同卤代烃的后聚合季铵化作用实现了表面疏水性的区分。通过定性和定量方法,包括比浊滴定法,动态光散射法和等温滴定量热法,研究了这些MNP与β-乳球蛋白(BLG)的离子强度和疏水性响应结合。从MNP–BLG络合的临界结合pH和结合常数判断,结合亲和力对表面疏水性的依赖性表现出随着离子强度的增加而发生的有趣变化,这意味着具有较高表面疏水性的MNP在较低离子强度下表现出较弱的结合亲和力,而在较高离子强度下表现出较强的亲和力。这种有趣的观察结果可以归因于离子强度对疏水和静电相互作用的响应能力的差异。通过这种方式,可以通过控制MNP的表面疏水性和离子强度,以最佳的结合亲和力实现良好的结合模式,从而赋予该系统很大的潜力,使其具有高选择性和高效率地制备分离和递送系统。这意味着具有较高表面疏水性的MNP在较低的离子强度下表现出较弱的结合亲和力,而在较高的离子强度下表现出较强的亲和力。这种有趣的观察结果可以归因于离子强度对疏水和静电相互作用的响应能力的差异。通过这种方式,可以通过控制MNP的表面疏水性和离子强度,以最佳的结合亲和力实现良好的结合模式,从而赋予该系统很大的潜力,使其具有高选择性和高效率地制备分离和递送系统。这意味着具有较高表面疏水性的MNP在较低的离子强度下表现出较弱的结合亲和力,而在较高的离子强度下表现出较强的亲和力。这种有趣的观察结果可以归因于离子强度对疏水和静电相互作用的响应能力的差异。通过这种方式,可以通过控制MNP的表面疏水性和离子强度,以最佳的结合亲和力实现良好的结合模式,从而赋予该系统很大的潜力,使其具有高选择性和高效率地制备分离和递送系统。这种有趣的观察结果可以归因于离子强度对疏水和静电相互作用的响应能力的差异。通过这种方式,可以通过控制MNP的表面疏水性和离子强度,以最佳的结合亲和力实现良好的结合模式,从而赋予该系统很大的潜力,使其具有高选择性和高效率地制备分离和递送系统。这种有趣的观察结果可以归因于离子强度对疏水和静电相互作用的响应能力的差异。通过这种方式,可以通过控制MNP的表面疏水性和离子强度,以最佳的结合亲和力实现良好的结合模式,从而赋予该系统很大的潜力,使其具有高选择性和高效率地制备分离和递送系统。
更新日期:2018-06-22
中文翻译:

离子强度响应纳米粒子和蛋白质之间的绑定。
静电相互作用是一种强大的,占优势的非特异性相互作用,已在蛋白质-纳米颗粒(NP)相互作用中进行了广泛研究[











































 京公网安备 11010802027423号
京公网安备 11010802027423号