当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tyrosine Sulfation Restricts the Conformational Ensemble of a Flexible Peptide, Strengthening the Binding Affinity for an Antibody
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-23 00:00:00 , DOI: 10.1021/acs.biochem.8b00592 Kazuhiro Miyanabe 1 , Takefumi Yamashita 2 , Yoshito Abe 3 , Hiroki Akiba 4, 5 , Yuichiro Takamatsu 6 , Makoto Nakakido 4 , Takao Hamakubo 6 , Tadashi Ueda 3 , Jose M. M. Caaveiro 7 , Kouhei Tsumoto 1, 4, 5, 8
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-23 00:00:00 , DOI: 10.1021/acs.biochem.8b00592 Kazuhiro Miyanabe 1 , Takefumi Yamashita 2 , Yoshito Abe 3 , Hiroki Akiba 4, 5 , Yuichiro Takamatsu 6 , Makoto Nakakido 4 , Takao Hamakubo 6 , Tadashi Ueda 3 , Jose M. M. Caaveiro 7 , Kouhei Tsumoto 1, 4, 5, 8
Affiliation
|
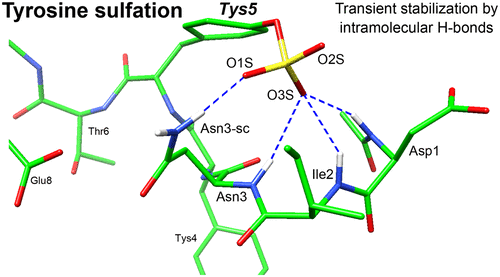
Protein tyrosine sulfation (PTS) is a post-translational modification regulating numerous biological events. PTS generally occurs at flexible regions of proteins, enhancing intermolecular interactions between proteins. Because of the high flexibility associated with the regions where PTS is generally encountered, an atomic-level understanding has been difficult to achieve by X-ray crystallography or nuclear magnetic resonance techniques. In this study, we focused on the conformational behavior of a flexible sulfated peptide and its interaction with an antibody. Molecular dynamics simulations and thermodynamic analysis indicated that PTS reduced the main-chain fluctuations upon the appearance of sulfate-mediated intramolecular H-bonds. Collectively, our data suggested that one of the mechanisms by which PTS may enhance protein–protein interactions consists of the limitation of conformational dynamics in the unbound state, thus reducing the loss of entropy upon binding and boosting the affinity for its partner.
中文翻译:

酪氨酸硫酸化限制了柔性肽的构象集合,增强了抗体的结合亲和力
蛋白质酪氨酸硫酸化(PTS)是一种翻译后修饰,可调节许多生物学事件。PTS通常发生在蛋白质的柔性区域,从而增强蛋白质之间的分子间相互作用。由于通常遇到PTS的区域具有很高的灵活性,因此通过X射线晶体学或核磁共振技术很难实现原子级的理解。在这项研究中,我们专注于柔性硫酸化肽的构象行为及其与抗体的相互作用。分子动力学模拟和热力学分析表明,PTS减少了硫酸盐介导的分子内H键出现时的主链波动。总的来说,
更新日期:2018-06-23
中文翻译:

酪氨酸硫酸化限制了柔性肽的构象集合,增强了抗体的结合亲和力
蛋白质酪氨酸硫酸化(PTS)是一种翻译后修饰,可调节许多生物学事件。PTS通常发生在蛋白质的柔性区域,从而增强蛋白质之间的分子间相互作用。由于通常遇到PTS的区域具有很高的灵活性,因此通过X射线晶体学或核磁共振技术很难实现原子级的理解。在这项研究中,我们专注于柔性硫酸化肽的构象行为及其与抗体的相互作用。分子动力学模拟和热力学分析表明,PTS减少了硫酸盐介导的分子内H键出现时的主链波动。总的来说,











































 京公网安备 11010802027423号
京公网安备 11010802027423号