Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2018-06-23 , DOI: 10.1016/j.cis.2018.06.003 Wanda Barzyk , Klaus Lunkenheimer , Andrzej Pomianowski
|
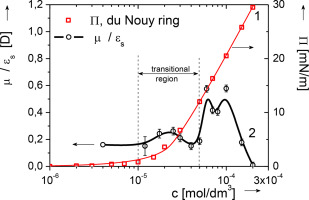
The surface pressure (Π) and electric surface potential (ΔV) vs. concentration (c) isotherms of n-decanoic acid (DA) in 1 × 10−3 mol/dm3 HCl were measured at the air/solution interface - the Π with a du Noüy ring, the ΔV with the vibrating plate (named also the dynamic condenser) method. The DA solutions fulfilled criterion of surface-chemical purity. The complementary Π-c and ΔV-c isotherms were jointly evaluated to obtain dependence of quotient of the effective dipole moment to the interface's permittivity, μ⊥/εs, as a function of chosen adsorption ordinates such as the bulk concentration, c, partial molar area, A, and surface molar fraction of DA, XDAs. The crucial point for the analysis is knowledge of the surface excess (Γ) dependence on concentration (c). Since, experimental determination of a Γ-c course is problematic, so far, we used Γ-c courses calculated basing on different adsorption models (Gibbsian, the classical Frumkin’ model and the Lunkenheimer’ and Hirte’ two state approach). Despite, the Γ-c courses determined basing on the different adsorption models differ significantly, the μ⊥/εs dependences on different adsorption's ordinates (c, A or XDAs) revealed consistently three local μ⊥/εs maxima of their height increasing with the adsorption coverage. The μ⊥/εs change was recalculated into inclination angle (αincl) of the total dipole moment vector () to the interface, assuming εs = 1. The μ⊥/εs which reflects polarization orientation is an order parameter used by us for analysis of 2D phase transitions in the monolayer. The three μ⊥/εs local maxima are ascribed by us to three 2D mono-phases, one transferring into the next one of the higher order in the sequence: liquid expanded (L1) → the liquid condensed tilted (L2) → the liquid condensed untilted (L2′). One inflection point appearing in the Π-A isotherm within the region between the μ⊥/εs maxima 2 and 3 indicates that transition of the L2 into the L2’ 2D phase is of the first order. Decrease of the μ⊥/εs above the maximum 3 indicates transition into two phase regime by nucleation of aggregates (possibly in form of lamellas) within the L2′ phase.
中文翻译:

通过表面压力和电势揭示的未离解正癸酸在空气/溶液界面的取向相变
在空气/溶液界面上测量正癸酸(DA)在1×10 -3 mol / dm 3 HCl中的表面压力(Π)和表面电势(ΔV)相对于浓度(c)等温线-Π如果使用duNoüy环,则采用振动板(也称为动态冷凝器)方法的ΔV。DA溶液满足表面化学纯度的标准。互补Π-c和ΔV-C等温线共同评估,以获得有效的偶极矩的商数的依赖于界面的介电常数,μ ⊥ /ε小号,作为选择吸附纵坐标的函数,如散装浓度c,部分摩尔面积A和DA的表面摩尔分数X DA s。分析的关键点是了解表面过量(Γ)对浓度(c)的依赖性。由于Γ-c过程的实验确定是有问题的,因此到目前为止,我们使用了基于不同吸附模型(吉布斯模型,经典Frumkin模型和Lunkenheimer'和Hirte'两种状态方法)计算的Γ-c过程。尽管,在Γ-C课程确定基础上的不同吸附模型显著不同,则μ ⊥ /ε小号上不同吸附的坐标(C,A或依赖性X DA小号)揭示一致三个本地μ ⊥ /ε小号它们的高度的最大值随着吸附覆盖率的增加。所述μ ⊥ /ε小号变化换算成倾斜角度(α含总偶极矩向量的)()到接口,假设ε小号 = 1μ ⊥ /ε小号反映偏振取向被用于单层2D相变的分析中使用由我们的命令参数。我们将这三个μ⊥ /εs的局部最大值归结为三个2D单相,一个按顺序转移到下一个更高阶的相中:液体膨胀(L1)→液体倾斜倾斜(L2)→液体浓缩直到(L2')。所述μ之间的区域内出现的Π-A等温线一个拐点⊥ /ε小号最大值2和3表示L2进入L2' 2D相过渡是一阶。μ⊥ /εs的减小 高于最大值3表示通过L2'相内的聚集体(可能以薄片形式)成核而转变为两相态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号