当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiazole-Induced Quinoid Polymers for Efficient Solar Cells: Influence of Molecular Skeleton, Regioselectivity, and Regioregularity
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.chemmater.8b01250 Dangqiang Zhu 1 , Qian Wang 1 , Yingying Wang 1 , Xichang Bao 1 , Meng Qiu 1 , Bilal Shahid 1 , Yonghai Li 1 , Renqiang Yang 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.chemmater.8b01250 Dangqiang Zhu 1 , Qian Wang 1 , Yingying Wang 1 , Xichang Bao 1 , Meng Qiu 1 , Bilal Shahid 1 , Yonghai Li 1 , Renqiang Yang 1
Affiliation
|
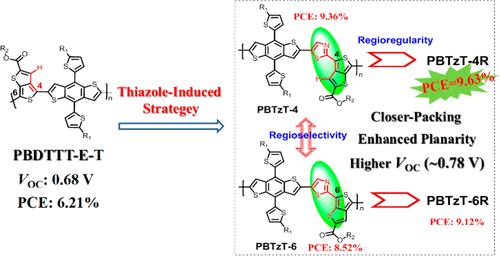
Fluorination strategy has been regarded as a promising approach to improve the photovoltaic performance in polymer solar cells. However, the synthesis is relatively tedious and costly for most fluorinated momomers. In this work, which is different from works where fluorine atoms are usually incorporated on the side chain, we successfully developed a thiazole-induced strategy to construct efficient photovoltaic materials via inserting one thiazole unit into the backbone of a nonfluorinated quinoid polymer, which would enhance the intermolecular interactions and decrease the ionization potential (IP) of the resulted polymers, benefiting from the desirable molecular skeleton. And then, considering the asymmetry nature of acceptor segments, the influence of molecular regioselectivity with different orientations of the thiazole unit on optoelectronic properties was systematically investigated. Encouragingly, a superior power conversion efficiency (PCE) of 9.36% for PBTzT-4-based photovoltaic device was obtained, higher than that of the isomer polymer PBTzT-6 (PCE = 8.52%) and a signficant increase of 50% compared to the widely reported analogue polymer PBDTTT-E-T (PCE = 6.21%) just without a thiazole unit, which can be ascribed to more planar molecular conformation, stronger crystallinity, and excellent phase separation. More interestingly, compared with random polymers, the regioregular copolymers exhibit enhanced red-shifted absorption and better crystallinity and compatibility with PC71BM, leading to more desirable efficicency for PBTzT-4R-based devices (PCE = 9.63%) with higher JSC of 17.56 mA/cm2, which can be comparable to the typical polymer PTB7-Th. This work not only provides a new strategy to improve the intermolecular interaction through backbone design but also reveals that the orientations of the asymmetric unit (that is, regioselectivity and regioregularity) along the polymer backbone play a crucial role and should be taken into account in future molecule design.
中文翻译:

噻唑诱导的高效太阳能醌聚合物:分子骨架,区域选择性和区域规则性的影响
氟化策略已被认为是改善聚合物太阳能电池中光伏性能的一种有前途的方法。然而,对于大多数氟化单体而言,合成相对繁琐且昂贵。在这项与通常在侧链上掺入氟原子的工作不同的是,我们成功地开发了噻唑诱导的策略,通过将一个噻唑单元插入非氟化醌类聚合物的骨架来构建有效的光伏材料。得益于理想的分子骨架,分子间的相互作用降低了所得聚合物的电离电势(IP)。然后,考虑到受体链段的不对称性,系统地研究了噻唑单元不同取向的分子区域选择性对光电性能的影响。令人鼓舞的是,对于基于PBTzT-4的光伏器件,获得了9.36%的出色功率转换效率(PCE),高于异构体聚合物PBTzT-6(PCE = 8.52%),并且与PBTzT-6异构体聚合物PBTzT-6相比,显着提高了50%。没有噻唑单元的广泛报道的类似聚合物PBDTTT-ET(PCE = 6.21%),可以归因于更平面的分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 与基于PBTzT-4的光伏器件相比,其功率转换效率(PCE)为9.36%,高于异构体聚合物PBTzT-6(PCE = 8.52%),与广泛报道的相比,显着提高了50%不含噻唑单元的类似聚合物PBDTTT-ET(PCE = 6.21%),可以归因于更平面的分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 与基于PBTzT-4的光伏器件相比,其功率转换效率(PCE)为9.36%,高于异构体聚合物PBTzT-6(PCE = 8.52%),与广泛报道的相比,显着提高了50%不含噻唑单元的类似聚合物PBDTTT-ET(PCE = 6.21%),可以归因于更平面的分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 可以归因于更多的平面分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 可以归因于更多的平面分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性71 BM,导致具有更高的J SC为17.56 mA / cm 2的基于PBTzT-4R的器件(PCE = 9.63%)具有更高的效率,这可以与典型的聚合物PTB7-Th相媲美。这项工作不仅提供了通过主链设计改善分子间相互作用的新策略,而且还揭示了沿聚合物主链的不对称单元的取向(即区域选择性和区域规则性)起着至关重要的作用,今后应予以考虑。分子设计。
更新日期:2018-06-22
中文翻译:

噻唑诱导的高效太阳能醌聚合物:分子骨架,区域选择性和区域规则性的影响
氟化策略已被认为是改善聚合物太阳能电池中光伏性能的一种有前途的方法。然而,对于大多数氟化单体而言,合成相对繁琐且昂贵。在这项与通常在侧链上掺入氟原子的工作不同的是,我们成功地开发了噻唑诱导的策略,通过将一个噻唑单元插入非氟化醌类聚合物的骨架来构建有效的光伏材料。得益于理想的分子骨架,分子间的相互作用降低了所得聚合物的电离电势(IP)。然后,考虑到受体链段的不对称性,系统地研究了噻唑单元不同取向的分子区域选择性对光电性能的影响。令人鼓舞的是,对于基于PBTzT-4的光伏器件,获得了9.36%的出色功率转换效率(PCE),高于异构体聚合物PBTzT-6(PCE = 8.52%),并且与PBTzT-6异构体聚合物PBTzT-6相比,显着提高了50%。没有噻唑单元的广泛报道的类似聚合物PBDTTT-ET(PCE = 6.21%),可以归因于更平面的分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 与基于PBTzT-4的光伏器件相比,其功率转换效率(PCE)为9.36%,高于异构体聚合物PBTzT-6(PCE = 8.52%),与广泛报道的相比,显着提高了50%不含噻唑单元的类似聚合物PBDTTT-ET(PCE = 6.21%),可以归因于更平面的分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 与基于PBTzT-4的光伏器件相比,其功率转换效率(PCE)为9.36%,高于异构体聚合物PBTzT-6(PCE = 8.52%),与广泛报道的相比,显着提高了50%不含噻唑单元的类似聚合物PBDTTT-ET(PCE = 6.21%),可以归因于更平面的分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 可以归因于更多的平面分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性 可以归因于更多的平面分子构象,更强的结晶度和出色的相分离。更有趣的是,与无规聚合物相比,区域规则共聚物表现出增强的红移吸收性,更好的结晶度以及与PC的相容性71 BM,导致具有更高的J SC为17.56 mA / cm 2的基于PBTzT-4R的器件(PCE = 9.63%)具有更高的效率,这可以与典型的聚合物PTB7-Th相媲美。这项工作不仅提供了通过主链设计改善分子间相互作用的新策略,而且还揭示了沿聚合物主链的不对称单元的取向(即区域选择性和区域规则性)起着至关重要的作用,今后应予以考虑。分子设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号