当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dissecting Substrate Specificities of the Mitochondrial AFG3L2 Protease
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.biochem.8b00565 Bojian Ding , Dwight W. Martin , Anthony J. Rampello , Steven E. Glynn
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.biochem.8b00565 Bojian Ding , Dwight W. Martin , Anthony J. Rampello , Steven E. Glynn
|
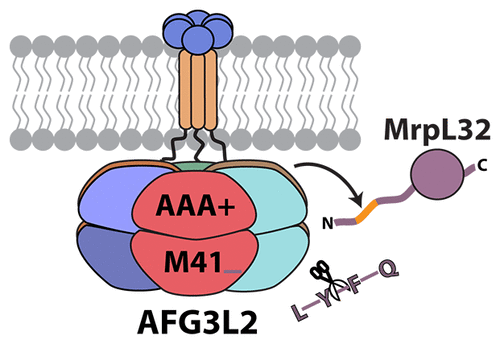
Human AFG3L2 is a compartmental AAA+ protease that performs ATP-fueled degradation at the matrix face of the inner mitochondrial membrane. Identifying how AFG3L2 selects substrates from the diverse complement of matrix-localized proteins is essential for understanding mitochondrial protein biogenesis and quality control. Here, we create solubilized forms of AFG3L2 to examine the enzyme’s substrate specificity mechanisms. We show that conserved residues within the presequence of the mitochondrial ribosomal protein, MrpL32, target the subunit to the protease for processing into a mature form. Moreover, these residues can act as a degron, delivering diverse model proteins to AFG3L2 for degradation. By determining the sequence of degradation products from multiple substrates using mass spectrometry, we construct a peptidase specificity profile that displays constrained product lengths and is dominated by the identity of the residue at the P1′ position, with a strong preference for hydrophobic and small polar residues. This specificity profile is validated by examining the cleavage of both fluorogenic reporter peptides and full polypeptide substrates bearing different P1′ residues. Together, these results demonstrate that AFG3L2 contains multiple modes of specificity, discriminating between potential substrates by recognizing accessible degron sequences and performing peptide bond cleavage at preferred patterns of residues within the compartmental chamber.
中文翻译:

解剖线粒体AFG3L2蛋白酶的底物特异性
人AFG3L2是一种隔室AAA +蛋白酶,可在内部线粒体膜的基质表面执行由ATP刺激的降解。鉴定AFG3L2如何从基质定位蛋白的多样化补体中选择底物对于理解线粒体蛋白的生物发生和质量控制至关重要。在这里,我们创建了AFG3L2的增溶形式来检查酶的底物特异性机制。我们显示线粒体核糖体蛋白,MrpL32,presequence内的保守残基靶向该亚基的蛋白酶加工成成熟形式。此外,这些残基可以充当德贡,将各种模型蛋白传递至AFG3L2进行降解。通过使用质谱确定来自多种底物的降解产物的顺序,我们构建了一种肽酶特异性谱,该谱显示了受限制的产物长度,并受P1'位置残基的身份支配,强烈偏爱疏水残基和小的极性残基。通过检查荧光报道肽和带有不同P1'残基的完整多肽底物的裂解,可以验证这种特异性。在一起,这些结果表明,AFG3L2包含多种特异性模式,通过识别可及的德格隆序列并在隔室中优选的残基模式进行肽键裂解来区分潜在的底物。通过检查荧光报道肽和带有不同P1'残基的完整多肽底物的裂解,可以验证这种特异性。在一起,这些结果表明,AFG3L2包含多种特异性模式,通过识别可及的德格隆序列并在隔室中优选的残基模式进行肽键裂解来区分潜在的底物。通过检查荧光报道肽和带有不同P1'残基的完整多肽底物的裂解,可以验证这种特异性。在一起,这些结果表明,AFG3L2包含多种特异性模式,通过识别可及的德格隆序列并在隔室中优选的残基模式进行肽键裂解来区分潜在的底物。
更新日期:2018-06-22
中文翻译:

解剖线粒体AFG3L2蛋白酶的底物特异性
人AFG3L2是一种隔室AAA +蛋白酶,可在内部线粒体膜的基质表面执行由ATP刺激的降解。鉴定AFG3L2如何从基质定位蛋白的多样化补体中选择底物对于理解线粒体蛋白的生物发生和质量控制至关重要。在这里,我们创建了AFG3L2的增溶形式来检查酶的底物特异性机制。我们显示线粒体核糖体蛋白,MrpL32,presequence内的保守残基靶向该亚基的蛋白酶加工成成熟形式。此外,这些残基可以充当德贡,将各种模型蛋白传递至AFG3L2进行降解。通过使用质谱确定来自多种底物的降解产物的顺序,我们构建了一种肽酶特异性谱,该谱显示了受限制的产物长度,并受P1'位置残基的身份支配,强烈偏爱疏水残基和小的极性残基。通过检查荧光报道肽和带有不同P1'残基的完整多肽底物的裂解,可以验证这种特异性。在一起,这些结果表明,AFG3L2包含多种特异性模式,通过识别可及的德格隆序列并在隔室中优选的残基模式进行肽键裂解来区分潜在的底物。通过检查荧光报道肽和带有不同P1'残基的完整多肽底物的裂解,可以验证这种特异性。在一起,这些结果表明,AFG3L2包含多种特异性模式,通过识别可及的德格隆序列并在隔室中优选的残基模式进行肽键裂解来区分潜在的底物。通过检查荧光报道肽和带有不同P1'残基的完整多肽底物的裂解,可以验证这种特异性。在一起,这些结果表明,AFG3L2包含多种特异性模式,通过识别可及的德格隆序列并在隔室中优选的残基模式进行肽键裂解来区分潜在的底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号