当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-Atomic Cu with Multiple Oxygen Vacancies on Ceria for Electrocatalytic CO2 Reduction to CH4
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acscatal.8b01014 Yifei Wang 1 , Zheng Chen 1 , Peng Han 1 , Yonghua Du 2 , Zhengxiang Gu 1 , Xin Xu 1 , Gengfeng Zheng 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acscatal.8b01014 Yifei Wang 1 , Zheng Chen 1 , Peng Han 1 , Yonghua Du 2 , Zhengxiang Gu 1 , Xin Xu 1 , Gengfeng Zheng 1
Affiliation
|
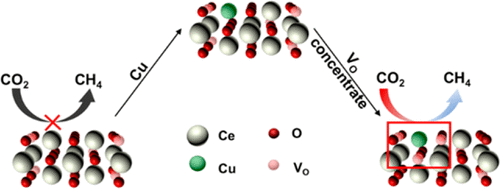
The electrocatalytic reduction of CO2 into value-added chemicals such as hydrocarbons has the potential for supplying fuel energy and reducing environmental hazards, while the accurate tuning of electrocatalysts at the ultimate single-atomic level remains extremely challenging. In this work, we demonstrate an atomic design of multiple oxygen vacancy-bound, single-atomic Cu-substituted CeO2 to optimize the CO2 electrocatalytic reduction to CH4. We carried out theoretical calculations to predict that the single-atomic Cu substitution in CeO2(110) surface can stably enrich up to three oxygen vacancies around each Cu site, yielding a highly effective catalytic center for CO2 adsorption and activation. This theoretical prediction is consistent with our controlled synthesis of the Cu-doped, mesoporous CeO2 nanorods. Structural characterizations indicate that the low concentration (<5%) Cu species in CeO2 nanorods are highly dispersed at single-atomic level with an unconventionally low coordination number ∼5, suggesting the direct association of 3 oxygen vacancies with each Cu ion on surfaces. This multiple oxygen vacancy-bound, single atomic Cu-substituted CeO2 enables an excellent electrocatalytic selectivity in reducing CO2 to methane with a faradaic efficiency as high as 58%, suggesting strong capabilities of rational design of electrocatalyst active centers for boosting activity and selectivity.
中文翻译:

二氧化铈上具有多个氧空位的单原子铜将CO 2电催化还原成CH 4
将CO 2电催化还原为增值化学品(例如碳氢化合物)具有提供燃料能源和减少环境危害的潜力,而在最终单原子水平上精确调节电催化剂仍然极具挑战性。在这项工作中,我们演示了多个氧空位结合的,单原子的Cu取代的CeO 2的原子设计,以优化CO 2电催化还原为CH 4。我们进行了理论计算,以预测CeO 2(110)表面的单原子Cu取代可以稳定地富集每个Cu位点周围的三个氧空位,从而产生高效的CO 2催化中心吸附和活化。该理论预测与我们对铜掺杂的介孔CeO 2纳米棒的受控合成相一致。结构表征表明,CeO 2纳米棒中的低浓度(<5%)Cu物种以单原子水平高度分散,且配位数低至〜5,这暗示了3个氧空位与表面上的每个Cu离子直接缔合。这种与多个氧空位结合的单原子Cu取代的CeO 2具有极好的电催化选择性,可将CO 2还原为甲烷,法拉第效率高达58%,这暗示了合理设计电催化剂活性中心以提高活性和选择性的强大能力。 。
更新日期:2018-06-22
中文翻译:

二氧化铈上具有多个氧空位的单原子铜将CO 2电催化还原成CH 4
将CO 2电催化还原为增值化学品(例如碳氢化合物)具有提供燃料能源和减少环境危害的潜力,而在最终单原子水平上精确调节电催化剂仍然极具挑战性。在这项工作中,我们演示了多个氧空位结合的,单原子的Cu取代的CeO 2的原子设计,以优化CO 2电催化还原为CH 4。我们进行了理论计算,以预测CeO 2(110)表面的单原子Cu取代可以稳定地富集每个Cu位点周围的三个氧空位,从而产生高效的CO 2催化中心吸附和活化。该理论预测与我们对铜掺杂的介孔CeO 2纳米棒的受控合成相一致。结构表征表明,CeO 2纳米棒中的低浓度(<5%)Cu物种以单原子水平高度分散,且配位数低至〜5,这暗示了3个氧空位与表面上的每个Cu离子直接缔合。这种与多个氧空位结合的单原子Cu取代的CeO 2具有极好的电催化选择性,可将CO 2还原为甲烷,法拉第效率高达58%,这暗示了合理设计电催化剂活性中心以提高活性和选择性的强大能力。 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号