当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of the Nature of the Amino Group in Highly Fluorescent Difluoroborates Exhibiting Intramolecular Charge Transfer
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.joc.8b00664 Beata Jędrzejewska 1 , Agnieszka Skotnicka 1 , Adèle D. Laurent 2 , Marek Pietrzak 1 , Denis Jacquemin 2 , Borys Ośmiałowski 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1021/acs.joc.8b00664 Beata Jędrzejewska 1 , Agnieszka Skotnicka 1 , Adèle D. Laurent 2 , Marek Pietrzak 1 , Denis Jacquemin 2 , Borys Ośmiałowski 3
Affiliation
|
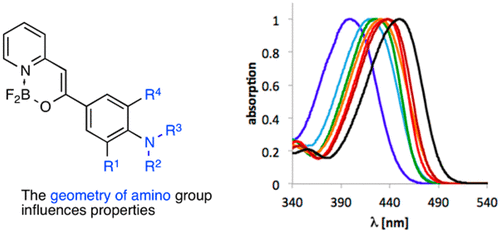
A series of difluoroborates were synthesized from CH acids. All compounds were substituted with dialkylamino groups (NR2). The lone electron pair of the nitrogen atom of this donor moiety is variably delocalized toward the difluoroborate core that acts as the electron acceptor. This was rationalized in light of the various geometries of the amino group. The degree of charge transfer was quantified on the basis of the results of time-dependent density functional theory calculations.
中文翻译:

展示分子内电荷转移的高荧光二氟硼酸盐中氨基基团性质的影响
由CH酸合成一系列二氟硼酸盐。所有化合物均被二烷基氨基基团(NR 2)取代。该供体部分的氮原子的孤电子对可变地偏向充当电子受体的二氟硼酸酯核。考虑到氨基的各种几何形状,这是合理的。基于随时间变化的密度泛函理论计算的结果来量化电荷转移的程度。
更新日期:2018-06-22
中文翻译:

展示分子内电荷转移的高荧光二氟硼酸盐中氨基基团性质的影响
由CH酸合成一系列二氟硼酸盐。所有化合物均被二烷基氨基基团(NR 2)取代。该供体部分的氮原子的孤电子对可变地偏向充当电子受体的二氟硼酸酯核。考虑到氨基的各种几何形状,这是合理的。基于随时间变化的密度泛函理论计算的结果来量化电荷转移的程度。









































 京公网安备 11010802027423号
京公网安备 11010802027423号