当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the mechanism of transition metal-free anti addition to alkynes: the selenoboration case†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c7cy02295f Diego García-López 1, 2, 3, 4 , Marc G. Civit 1, 2, 3, 4 , Christopher M. Vogels 5, 6, 7, 8 , Josep M. Ricart 1, 2, 3, 4 , Stephen A. Westcott 5, 6, 7, 8 , Elena Fernández 1, 2, 3, 4 , Jorge J. Carbó 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c7cy02295f Diego García-López 1, 2, 3, 4 , Marc G. Civit 1, 2, 3, 4 , Christopher M. Vogels 5, 6, 7, 8 , Josep M. Ricart 1, 2, 3, 4 , Stephen A. Westcott 5, 6, 7, 8 , Elena Fernández 1, 2, 3, 4 , Jorge J. Carbó 1, 2, 3, 4
Affiliation
|
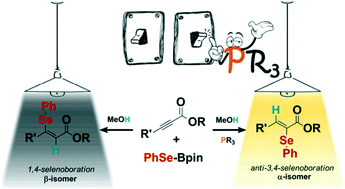
The stereoselective anti-addition of selenoboranes to α,β-acetylenic esters and amides was achieved in a transition metal-free context using catalytic amounts of PCy3. The reaction provides anti-3,4-selenoboration with concomitant delivery of α-vinyl selenides by protodeboronation with MeOH. Interestingly, in the absence of phosphine the selenoboration switches towards the formation of β-vinyl selenides. Theoretical calculations rationalize the regio- and stereoselectivity of the reaction by discovering a new mechanism for the anti-3,4-selenoboration. While the selenoborane is activated via the “push–pull” effect of B, the phosphine interacts with the β position of the alkynoate switching the polarity of the triple bond and favoring 1,3-selenoboration which provides the α-addition of the selenyl group. Then, the autocatalytic action of a second selenoborane reagent, which coordinates to the phosphorus ylide intermediate, determines the stereoselectivity and completes the catalytic process. Finally, the comparison of selenoborane reagents with diboranes and silaboranes, which have exhibited analogous reactivity, shows that the selenium moiety has a larger nucleophilic character favoring the performance of the reaction under mild conditions.
中文翻译:

了解炔烃中无过渡金属的反加成机理:硒代硼烷情况†
在无过渡金属的情况下,使用催化量的PCy 3可实现硒代硼烷向α,β-炔属酸酯和酰胺的立体选择性抗加成反应。该反应提供了抗-3,4-硒代硼酸酯化,同时通过用MeOH进行原硼烷脱硼而递送了α-乙烯基硒化物。有趣的是,在不存在膦的情况下,硒代硼酸酯转变为β-乙烯基硒化物的形成。理论计算通过发现抗-3,4-硒代硼化的新机制合理化了反应的区域选择性和立体选择性。当硒硼烷通过在B的“推挽”效应下,膦与链烷酸酯的β位置相互作用,从而切换三键的极性并促进1,3-硒代硼酸酯化,从而提供硒烯基的α加成。然后,第二硒代硼烷试剂的自催化作用与磷酰叶内酯中间体配合,确定了立体选择性并完成了催化过程。最后,硒硼烷试剂与已显示类似反应性的乙硼烷和硅硼烷的比较表明,硒部分具有较大的亲核特性,有利于在温和条件下进行反应。
更新日期:2018-06-22
中文翻译:

了解炔烃中无过渡金属的反加成机理:硒代硼烷情况†
在无过渡金属的情况下,使用催化量的PCy 3可实现硒代硼烷向α,β-炔属酸酯和酰胺的立体选择性抗加成反应。该反应提供了抗-3,4-硒代硼酸酯化,同时通过用MeOH进行原硼烷脱硼而递送了α-乙烯基硒化物。有趣的是,在不存在膦的情况下,硒代硼酸酯转变为β-乙烯基硒化物的形成。理论计算通过发现抗-3,4-硒代硼化的新机制合理化了反应的区域选择性和立体选择性。当硒硼烷通过在B的“推挽”效应下,膦与链烷酸酯的β位置相互作用,从而切换三键的极性并促进1,3-硒代硼酸酯化,从而提供硒烯基的α加成。然后,第二硒代硼烷试剂的自催化作用与磷酰叶内酯中间体配合,确定了立体选择性并完成了催化过程。最后,硒硼烷试剂与已显示类似反应性的乙硼烷和硅硼烷的比较表明,硒部分具有较大的亲核特性,有利于在温和条件下进行反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号