当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
O-Substituted group-controlled selectivity in Rh(iii)-catalyzed coupling of benzamides with α,α-difluoromethylene alkynes: a computational mechanistic study†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c8cy00481a Xue-Xiang Ma 1, 2, 3, 4, 5 , Jian-Biao Liu 1, 2, 3, 4, 5 , Fang Huang 1, 2, 3, 4, 5 , Chuan-Zhi Sun 1, 2, 3, 4, 5 , De-Zhan Chen 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-06-22 00:00:00 , DOI: 10.1039/c8cy00481a Xue-Xiang Ma 1, 2, 3, 4, 5 , Jian-Biao Liu 1, 2, 3, 4, 5 , Fang Huang 1, 2, 3, 4, 5 , Chuan-Zhi Sun 1, 2, 3, 4, 5 , De-Zhan Chen 1, 2, 3, 4, 5
Affiliation
|
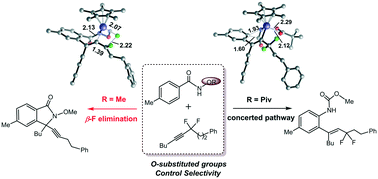
The Rh(III)-catalyzed C–H activation of benzamide derivatives with α,α-difluoromethylene alkynes was studied by DFT calculations to elucidate the selectivity controlled by different O-substituted groups (OPiv versus OMe). The reactions share a similar process that involves N–H deprotonation, C–H activation and alkyne insertion to form a 7-membered rhodacycle. Then the reaction pathway bifurcates for the two different directing groups. For OPiv, the following step involves concerted ipso attack and pivalate migration, methanol nucleophilic addition and protonation to generate hydroarylation products. For OMe, the subsequent pathway contains the first β-F elimination, intramolecular aminorhodation and the second β-F elimination to produce [4 + 1] annulation products. The N–O bond strength and the coordination of the O-substituted groups to Rh are responsible for the selectivity. The carbonyl oxygen in the OPiv group provides crucial rhodium–oxygen interaction, which essentially stabilizes the 7-membered intermediate and thus facilitates the concerted ipso attack and pivalate migration process, while the much stronger N–O bond of the OMe-containing 7-membered rhodacycle promotes the β-F elimination.
中文翻译:

苯甲酰胺与α,α-二氟亚甲基炔烃的Rh(iii)催化偶合中的O取代基受控选择性:计算机理研究†
通过DFT计算研究了用α,α-二氟亚甲基炔烃对Rh(III)催化的苯甲酰胺衍生物的C–H活化,以阐明不同O取代基(OPiv与OMe)控制的选择性。这些反应具有相似的过程,涉及N–H去质子化,C–H活化和炔烃插入以形成7元的Rhodacycle。然后,对于两个不同的导向基团,反应路径分叉。对于OPiv,下面的步骤包括协同IPSO攻击和新戊酸酯迁移,甲醇亲核加成和质子化生成氢芳基化产物。对于OMe,后续途径包含第一个β-F消除,分子内氨基铑化和第二个β-F消除,以产生[4 +1]环化产物。N–O键的强度和O取代基与Rh的配位决定了选择性。OPiv基团中的羰基氧提供了至关重要的铑-氧相互作用,从而基本稳定了7元中间体,从而促进了一致的ipso攻击和新戊酸酯迁移过程,而含OMe的7元中间体的N-O键更强罗丹环促进了β-F的消除。
更新日期:2018-06-22
中文翻译:

苯甲酰胺与α,α-二氟亚甲基炔烃的Rh(iii)催化偶合中的O取代基受控选择性:计算机理研究†
通过DFT计算研究了用α,α-二氟亚甲基炔烃对Rh(III)催化的苯甲酰胺衍生物的C–H活化,以阐明不同O取代基(OPiv与OMe)控制的选择性。这些反应具有相似的过程,涉及N–H去质子化,C–H活化和炔烃插入以形成7元的Rhodacycle。然后,对于两个不同的导向基团,反应路径分叉。对于OPiv,下面的步骤包括协同IPSO攻击和新戊酸酯迁移,甲醇亲核加成和质子化生成氢芳基化产物。对于OMe,后续途径包含第一个β-F消除,分子内氨基铑化和第二个β-F消除,以产生[4 +1]环化产物。N–O键的强度和O取代基与Rh的配位决定了选择性。OPiv基团中的羰基氧提供了至关重要的铑-氧相互作用,从而基本稳定了7元中间体,从而促进了一致的ipso攻击和新戊酸酯迁移过程,而含OMe的7元中间体的N-O键更强罗丹环促进了β-F的消除。










































 京公网安备 11010802027423号
京公网安备 11010802027423号