当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereocontrolled Monoprotodeboronation of β‐Sulfinimido gem‐Bis(boronates): A General and Stereoselective Route to α,β‐Disubstituted β‐Aminoalkylboronates
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-18 , DOI: 10.1002/anie.201804277 Xiangyu Li 1 , Dennis G. Hall 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-18 , DOI: 10.1002/anie.201804277 Xiangyu Li 1 , Dennis G. Hall 1
Affiliation
|
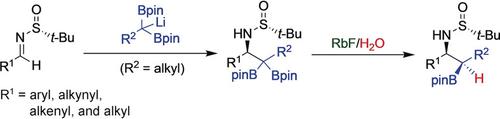
β‐Aminoalkylboronic acids are bioisosteres of the pharmaceutically important class of β‐amino acids but few stereoselective methods exist for their preparation. The 1,2‐addition of lithiated 1,1‐diborylalkanes onto chiral N‐tert‐butanesulfinyl aldimines produces β‐sulfinimido gem‐bis(boronates) in good to excellent yields with high diastereoselectivity. The optimized conditions involve the use of rubidium fluoride and water, and are compatible with functionalized alkyl, aryl, alkenyl, and alkynyl substituents. Under these conditions, the geminal quaternary alkyl bis(pinacolatoboryl) intermediates undergo a highly diastereoselective monoprotodeboronation to afford a wide range of syn‐α,β‐disubstituted β‐aminoalkylboronates. This novel application of protodeboronation chemistry was shown to result from a kinetically controlled, diastereotopic‐group‐selective B−C bond protolysis dictated by the configuration of the adjacent stereogenic C−N center. Facile acidic cleavage of the sulfinimide auxiliary produces the free aminoboronates with high enantiomeric purity.
中文翻译:

β-亚磺酰亚胺基gem-Bis(硼酸酯)的非对映控制的单原脱硼烷:α,β-二取代的β-氨基烷基硼酸酯的一般和立体选择路线
β-氨基烷基硼酸是药物上重要的β-氨基酸类的生物等排体,但很少有立体选择性的制备方法。1,2-加成锂化1,1- diborylalkanes的手性上ñ -叔-butanesulfinyl醛亚胺产生β-sulfinimido宝石-双良好(硼酸酯)以高非对映选择性优良的产率。优化的条件包括使用氟化rub和水,并且与官能化的烷基,芳基,烯基和炔基取代基相容。在这些条件下,双键季烷基双(频哪醇硼烷基)中间体经历高度非对映选择性的单原脱硼硼烷,以提供广泛的顺式-α,β-二取代的β-氨基烷基硼酸酯。原硼烷化化学的这种新应用被证明是由动力学控制的,非对映体-基团选择性的B-C键分解引起的,该分解由相邻的立体C-N中心的构型决定。磺酰亚胺酰亚胺助剂的容易的酸性裂解产生具有高对映体纯度的游离氨基硼酸酯。
更新日期:2018-07-18
中文翻译:

β-亚磺酰亚胺基gem-Bis(硼酸酯)的非对映控制的单原脱硼烷:α,β-二取代的β-氨基烷基硼酸酯的一般和立体选择路线
β-氨基烷基硼酸是药物上重要的β-氨基酸类的生物等排体,但很少有立体选择性的制备方法。1,2-加成锂化1,1- diborylalkanes的手性上ñ -叔-butanesulfinyl醛亚胺产生β-sulfinimido宝石-双良好(硼酸酯)以高非对映选择性优良的产率。优化的条件包括使用氟化rub和水,并且与官能化的烷基,芳基,烯基和炔基取代基相容。在这些条件下,双键季烷基双(频哪醇硼烷基)中间体经历高度非对映选择性的单原脱硼硼烷,以提供广泛的顺式-α,β-二取代的β-氨基烷基硼酸酯。原硼烷化化学的这种新应用被证明是由动力学控制的,非对映体-基团选择性的B-C键分解引起的,该分解由相邻的立体C-N中心的构型决定。磺酰亚胺酰亚胺助剂的容易的酸性裂解产生具有高对映体纯度的游离氨基硼酸酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号