当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Counterintuitive Interligand Angles in the Diaryls E{C6H3-2,6-(C6H2-2,4,6-iPr3)2}2 (E = Ge, Sn, or Pb) and Related Species: The Role of London Dispersion Forces
Organometallics ( IF 2.5 ) Pub Date : 2018-06-22 , DOI: 10.1021/acs.organomet.8b00225 Madison L. McCrea-Hendrick 1 , Markus Bursch 2 , Kelly L. Gullett 1 , Leonard R. Maurer 2 , James C. Fettinger 1 , Stefan Grimme 2 , Philip P. Power 1
Organometallics ( IF 2.5 ) Pub Date : 2018-06-22 , DOI: 10.1021/acs.organomet.8b00225 Madison L. McCrea-Hendrick 1 , Markus Bursch 2 , Kelly L. Gullett 1 , Leonard R. Maurer 2 , James C. Fettinger 1 , Stefan Grimme 2 , Philip P. Power 1
Affiliation
|
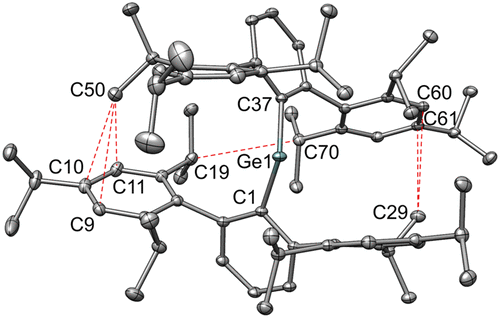
The straightforward reaction of two equivalents of the lithium salt of the bulky terphenyl ligand Li(OEt2)C6H3-2,6-(C6H2-2,4,6-iPr3)2 with suspensions of GeCl2·dioxane, SnCl2, or PbBr2 in diethyl ether resulted in the isolation of the very crowded σ-bonded diaryl tetrylenes of formula E{C6H3-2,6-(C6H2-2,4,6-iPr3)2}2 (E = Ge (1), Sn (2), Pb (3)) as blue crystalline solids. Despite their high level of steric congestion, X-ray crystallography showed that compounds 1–3 possess Cipso–E–Cipso interligand bond angles in the range 107.61–112.55°, which are narrower than those observed in analogous species with less bulky terphenyl substituents. Compounds 1–3 were characterized by 1H, 13C{1H} (1–3), and 119Sn{1H} (2) NMR spectroscopy, whereas solution 207Pb{1H} NMR spectroscopy of 3 has not yet afforded a signal under ambient conditions. FT-IR and UV–vis spectra of 1–3 were also recorded. The relatively narrow interligand angles displayed by 1–3 are attributed in part to the increase in London dispersion force interactions between the two AriPr6 (AriPr6 = -C6H3-2,6-(C6H2-2,4,6-iPr3)2) groups from carbon atoms in some of the isopropyl substituents and several carbon atoms from the flanking aryl rings. Density functional theory (DFT) calculations carried out at the PBE0/def2-QZVP level on the full series of diaryl tetrylenes, E(AriPr6)2, E(AriPr4)2 (AriPr4 = -C6H3-2,6-(C6H3-2,6-iPr2)2), and E(ArMe6)2 (ArMe6 = -C6H3-2,6-(C6H2-2,4,6-Me3)2, afford interaction energies as high as ca. 27 kcal mol–1.
中文翻译:

二芳基E {C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2 } 2(E = Ge,Sn或Pb)和相关物种中的反直觉配体角:伦敦分散力量的作用
两当量的大体积三联苯配体Li(OEt 2)C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2的锂盐与GeCl悬浮液的直接反应2 ·二恶烷,SnCl 2或PbBr 2在乙醚中的分离导致非常拥挤的式E {C 6 H 3 -2,6-(C 6 H 2 -2,4,6 -我镨3)2 } 2(E =葛(1),Sn(2),Pb(3))为蓝色结晶固体。尽管他们的高层次空间拥塞的,X射线结晶学表明,化合物1 - 3具有ç本位-E-C本位范围107.61-112.55 interligand键角°,它比在类似物种观察到的那些与体积较小的三联苯窄取代基。化合物13通过其特征在于1 H,13 C {1 1个H}(13),和119的Sn { 1个H}(2)NMR光谱,而溶液207的Pb { 1个的H} NMR光谱3还没有被环境条件下,得到的信号。FT-IR和UV-VIS光谱1 - 3也被记录。相对窄的角度interligand通过显示1 - 3是部分归因于在这两个之间的Ar分散力相互作用的增加我PR6(AR我PR6 = -C 6 H ^ 3 -2,6-(C 6 H ^ 2 -2 ,4,6- i Pr 3)2)来自一些异丙基取代基中的碳原子和来自侧基芳基环的几个碳原子。在全系列的二芳基四甲苯E(Ar i Pr6)2,E(Ar i Pr4)2(Ar i Pr4 = -C 6 H 3)的全系列中,以PBE0 / def2-QZVP级别进行密度泛函理论(DFT)计算-2,6-(C 6 H 3 -2,6- i Pr 2)2)和E(Ar Me6)2(Ar Me6 = -C 6 H 3 -2,6-(C 6H 2 -2,4,6-Me 3)2,提供高达约ca的相互作用能。27 kcal mol –1。
更新日期:2018-06-22
中文翻译:

二芳基E {C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2 } 2(E = Ge,Sn或Pb)和相关物种中的反直觉配体角:伦敦分散力量的作用
两当量的大体积三联苯配体Li(OEt 2)C 6 H 3 -2,6-(C 6 H 2 -2,4,6- i Pr 3)2的锂盐与GeCl悬浮液的直接反应2 ·二恶烷,SnCl 2或PbBr 2在乙醚中的分离导致非常拥挤的式E {C 6 H 3 -2,6-(C 6 H 2 -2,4,6 -我镨3)2 } 2(E =葛(1),Sn(2),Pb(3))为蓝色结晶固体。尽管他们的高层次空间拥塞的,X射线结晶学表明,化合物1 - 3具有ç本位-E-C本位范围107.61-112.55 interligand键角°,它比在类似物种观察到的那些与体积较小的三联苯窄取代基。化合物13通过其特征在于1 H,13 C {1 1个H}(13),和119的Sn { 1个H}(2)NMR光谱,而溶液207的Pb { 1个的H} NMR光谱3还没有被环境条件下,得到的信号。FT-IR和UV-VIS光谱1 - 3也被记录。相对窄的角度interligand通过显示1 - 3是部分归因于在这两个之间的Ar分散力相互作用的增加我PR6(AR我PR6 = -C 6 H ^ 3 -2,6-(C 6 H ^ 2 -2 ,4,6- i Pr 3)2)来自一些异丙基取代基中的碳原子和来自侧基芳基环的几个碳原子。在全系列的二芳基四甲苯E(Ar i Pr6)2,E(Ar i Pr4)2(Ar i Pr4 = -C 6 H 3)的全系列中,以PBE0 / def2-QZVP级别进行密度泛函理论(DFT)计算-2,6-(C 6 H 3 -2,6- i Pr 2)2)和E(Ar Me6)2(Ar Me6 = -C 6 H 3 -2,6-(C 6H 2 -2,4,6-Me 3)2,提供高达约ca的相互作用能。27 kcal mol –1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号