Journal of Catalysis ( IF 7.3 ) Pub Date : 2018-06-21 , DOI: 10.1016/j.jcat.2018.04.027 Danielle L.J. Pinheiro , Dennis U. Nielsen , Giovanni W. Amarante , Troels Skrydstrup
|
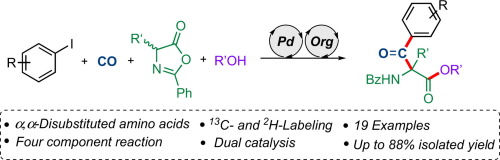
We report on a Pd-catalyzed carbonylative α-arylation of azlactones derived from alanine, providing facile access to α,α-disubstituted amino acids after an alcoholysis step. A range of aryl iodides proved to be suitable substrates in this formal four-component approach, providing the desired products in good to high yields. Other amino acids, such as methionine, leucine and phenylalanine could be functionalized in a similar manner with this methodology. The possibility for isotope labeling (13C, 2H) was demonstrated, as well as chemoselective transformations of the tricarbonyl-containing molecules.
中文翻译:

Pd催化的内酯羰基化α-芳基化:与α,α-二取代氨基酸形成正式的四组分偶联途径
我们报告了丙氨酸衍生的丙二酮的钯催化羰基化α-芳基化反应,提供了醇解步骤后对α,α-二取代氨基酸的便捷访问。在这种正式的四组分方法中,一系列芳基碘被证明是合适的底物,以高到高收率提供了所需的产物。其他氨基酸,例如蛋氨酸,亮氨酸和苯丙氨酸可以用这种方法以类似的方式进行功能化。证明了同位素标记(13 C,2 H)的可能性,以及含三羰基分子的化学选择性转化。




























 京公网安备 11010802027423号
京公网安备 11010802027423号