Chem ( IF 19.1 ) Pub Date : 2018-06-21 , DOI: 10.1016/j.chempr.2018.05.016 Ana M. Garcia , Daniel Iglesias , Evelina Parisi , Katie E. Styan , Lynne J. Waddington , Caterina Deganutti , Rita De Zorzi , Mario Grassi , Michele Melchionna , Attilio V. Vargiu , Silvia Marchesan
|
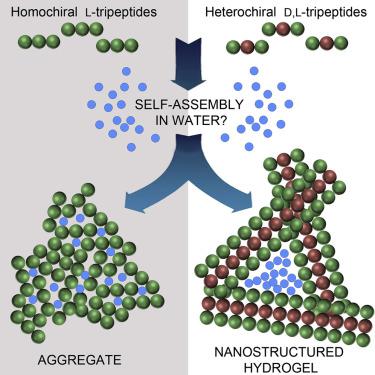
Self-assembling short peptides are attractive minimal systems for mimicking the constituents of living systems and building (bio)materials. The combination of both d- and l-amino acids into heterochiral sequences is a versatile strategy for building durable supramolecular architectures, especially when their homochiral analogs do not self-assemble. The reasons for this divergent behavior have remained obscure until now. Here, we elucidate how and why homochiral and heterochiral peptides behave differently. We identify a key spectroscopy signature and its corresponding molecular conformation, whereby an amphiphilic structure is uniquely enabled by the peptide stereochemistry. Importantly, we unravel the self-assembly process as a continuum from the conformation of single molecules to their organization into nano- and microstructures and through to macroscopic hydrogels, which are probed for cytotoxicity in fibroblast cell culture. In this way, (bio)material properties at the macro-scale can be linked to the chemical structure of their building blocks at the angstrom scale.
中文翻译:

手性对从分子到物质的肽自组装的影响
自组装短肽是用于模拟生命系统和建筑(生物)材料成分的极具吸引力的最小系统。d-和l的组合-将氨基酸变成杂手性序列是建立持久的超分子结构的一种通用策略,尤其是当它们的同手性类似物不能自组装时。迄今为止,这种不同行为的原因仍然不清楚。在这里,我们阐明同手性和异手性肽的行为方式和原因。我们确定了关键的光谱学签名及其相应的分子构象,从而通过肽立体化学独特地实现了两亲性结构。重要的是,我们揭示了自组装过程的连续性,即从单个分子的构象到其组织成纳米和微观结构,再到宏观水凝胶的连续过程,这些水凝胶可检测成纤维细胞培养中的细胞毒性。通过这种方式,











































 京公网安备 11010802027423号
京公网安备 11010802027423号