当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Control of Water Chemistry in Alkaline Lakes: Solubility of Monohydrocalcite and Amorphous Magnesium Carbonate in CaCl2–MgCl2–Na2CO3 Solutions
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00046 Keisuke Fukushi 1 , Haruna Matsumiya 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00046 Keisuke Fukushi 1 , Haruna Matsumiya 2
Affiliation
|
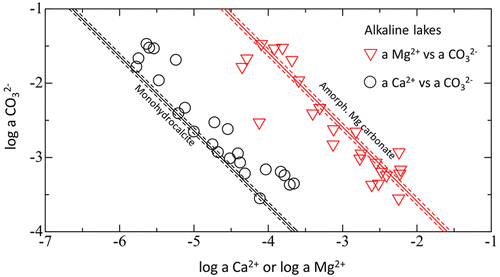
Alkaline lakes play an important role in the global carbon cycle. Nevertheless, little is known about the chemical processes related to sequestration of CO2 in these lakes. Our earlier study demonstrated that the formation of monohydrocalcite (MHC), a hydrous carbonate mineral frequently found in alkaline lakes, requires coexistence of amorphous Mg carbonate (AMC). It is therefore possible that MHC and AMC control the water chemistries of alkaline lakes. To test this hypothesis, we measured the solubilities of MHC and AMC in Na2CO3–MgCl2–CaCl2 solutions and compared them with the water chemistries of alkaline lakes. Results showed that the solubility of MHC is independent of the Mg contents of the system. The solubility product of AMC was almost 2 orders of magnitude higher than that of MHC. The water chemistries obtained from the alkaline saline lakes with pH > 9 around the world closely approximated saturation with respect to both MHC and AMC. MHC, which is a metastable phase, transforms quickly to aragonite or calcite. Under conditions in which MHC controls the solution chemistries, dissolved inorganic carbon is irreversibly and spontaneously isolated via formation of aragonite or calcite in the lakes. Results of this study suggest that alkaline lakes are natural devices for effective CO2 sequestration.
中文翻译:

碱性湖泊中水化学的控制:单氢方解石和无定形碳酸镁在CaCl 2 -MgCl 2 -Na 2 CO 3溶液中的溶解度
碱性湖泊在全球碳循环中起着重要作用。然而,对于这些湖泊中与固存CO 2有关的化学过程知之甚少。我们较早的研究表明,单水方解石(MHC)的形成(一种在碱性湖泊中经常发现的含水碳酸盐矿物),需要共存无定形的碳酸镁(AMC)。因此,MHC和AMC可能会控制碱性湖泊的水化学性质。为了验证这一假设,我们测量了MHC和AMC在Na 2 CO 3 –MgCl 2 –CaCl 2中的溶解度。解决方案,并将其与碱性湖泊的水化学性质进行比较。结果表明,MHC的溶解度与系统中Mg的含量无关。AMC的溶解度乘积比MHC高近2个数量级。从世界各地pH值大于9的碱性盐湖中获得的水化学成分,无论是MHC还是AMC都非常接近饱和度。MHC是亚稳相,可快速转变为文石或方解石。在MHC控制溶液化学性质的条件下,溶解的无机碳通过在湖中形成文石或方解石而不可逆转地自发分离。这项研究的结果表明,碱性湖泊是有效隔离CO 2的天然设备。
更新日期:2018-06-21
中文翻译:

碱性湖泊中水化学的控制:单氢方解石和无定形碳酸镁在CaCl 2 -MgCl 2 -Na 2 CO 3溶液中的溶解度
碱性湖泊在全球碳循环中起着重要作用。然而,对于这些湖泊中与固存CO 2有关的化学过程知之甚少。我们较早的研究表明,单水方解石(MHC)的形成(一种在碱性湖泊中经常发现的含水碳酸盐矿物),需要共存无定形的碳酸镁(AMC)。因此,MHC和AMC可能会控制碱性湖泊的水化学性质。为了验证这一假设,我们测量了MHC和AMC在Na 2 CO 3 –MgCl 2 –CaCl 2中的溶解度。解决方案,并将其与碱性湖泊的水化学性质进行比较。结果表明,MHC的溶解度与系统中Mg的含量无关。AMC的溶解度乘积比MHC高近2个数量级。从世界各地pH值大于9的碱性盐湖中获得的水化学成分,无论是MHC还是AMC都非常接近饱和度。MHC是亚稳相,可快速转变为文石或方解石。在MHC控制溶液化学性质的条件下,溶解的无机碳通过在湖中形成文石或方解石而不可逆转地自发分离。这项研究的结果表明,碱性湖泊是有效隔离CO 2的天然设备。



























 京公网安备 11010802027423号
京公网安备 11010802027423号