当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of Cs onto Biogenic Birnessite: Effects of Layer Structure, Ionic Strength, and Competition Cations
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00042 Qianqian Yu 1, 2 , Kazuya Tanaka 2 , Naofumi Kozai 2 , Fuminori Sakamoto 2 , Yukinori Tani 3 , Toshihiko Ohnuki 2, 4
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00042 Qianqian Yu 1, 2 , Kazuya Tanaka 2 , Naofumi Kozai 2 , Fuminori Sakamoto 2 , Yukinori Tani 3 , Toshihiko Ohnuki 2, 4
Affiliation
|
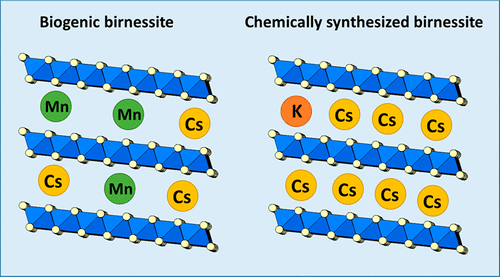
Although the adsorption of cesium (Cs) onto phyllosilicate minerals has been widely studied, the effect of Cs on redox-sensitive biogenic Mn oxide is not well understood. In this study, the structural transformation of biogenic birnessite stimulated by commonly occurring natural heavy metals, and the influence of those metals on the adsorption behavior of Cs over a wide range of concentrations (1 × 10–10 to 0.1 mol/L) was carefully examined via solution chemistry and X-ray absorption fine structure techniques. The Cs was reversibly adsorbed onto the mineral surface to form an outer-sphere coordination for all biogenic birnessite samples. The presence of heavy metals (e.g., Zn and Ni) during bio-oxidation of Mn(II), followed by acid treatment, increased the number of available layer vacancies, which consequently increased the adsorption capacity of Cs in the final product. The analysis of the dependence of sorption values on the ionic strength showed distinct results on biogenic birnessite and chemically synthesized birnessite. Trace ions (e.g., Mn2+) that were loosely bound to biogenic birnessite were released into the solution when they came in contact with the NaCl solution. The competition between these trace ions significantly influence Cs adsorption on biogenic birnessite. The results in this study indicated that it is necessary to account for the competition effect of trace ions, particularly when considering poorly crystalline adsorbents, such as biominerals.
中文翻译:

Cs吸附到生物水钠锰矿上:层结构,离子强度和竞争阳离子的影响。
尽管已经广泛研究了铯(Cs)在页硅酸盐矿物上的吸附,但是对铯对氧化还原敏感的生物型Mn氧化物的影响尚不十分清楚。在这项研究中,常见的天然重金属刺激了生物型水钠锰矿的结构转变,以及这些金属对宽浓度范围(1×10 –10)的Cs吸附行为的影响。通过溶液化学和X射线吸收精细结构技术仔细检查(0.1 mol / L)。Cs可逆地吸附在矿物表面上,从而形成了所有生物成水钠锰矿样品的外层配位。Mn(II)的生物氧化过程中重金属(例如Zn和Ni)的存在,然后进行酸处理,增加了可用层空位的数量,因此增加了最终产品中Cs的吸附能力。吸附值对离子强度的依赖性分析表明,生物成水钠锰矿和化学合成水钠锰矿具有明显的不同结果。痕量离子(例如Mn 2+与生物碱水钠锰矿松散结合的)当与NaCl溶液接触时被释放到溶液中。这些痕量离子之间的竞争会显着影响Cs在生物水钠锰矿上的吸附。这项研究的结果表明,有必要考虑痕量离子的竞争效应,尤其是在考虑结晶性较差的吸附剂(例如生物矿物)时。
更新日期:2018-06-21
中文翻译:

Cs吸附到生物水钠锰矿上:层结构,离子强度和竞争阳离子的影响。
尽管已经广泛研究了铯(Cs)在页硅酸盐矿物上的吸附,但是对铯对氧化还原敏感的生物型Mn氧化物的影响尚不十分清楚。在这项研究中,常见的天然重金属刺激了生物型水钠锰矿的结构转变,以及这些金属对宽浓度范围(1×10 –10)的Cs吸附行为的影响。通过溶液化学和X射线吸收精细结构技术仔细检查(0.1 mol / L)。Cs可逆地吸附在矿物表面上,从而形成了所有生物成水钠锰矿样品的外层配位。Mn(II)的生物氧化过程中重金属(例如Zn和Ni)的存在,然后进行酸处理,增加了可用层空位的数量,因此增加了最终产品中Cs的吸附能力。吸附值对离子强度的依赖性分析表明,生物成水钠锰矿和化学合成水钠锰矿具有明显的不同结果。痕量离子(例如Mn 2+与生物碱水钠锰矿松散结合的)当与NaCl溶液接触时被释放到溶液中。这些痕量离子之间的竞争会显着影响Cs在生物水钠锰矿上的吸附。这项研究的结果表明,有必要考虑痕量离子的竞争效应,尤其是在考虑结晶性较差的吸附剂(例如生物矿物)时。











































 京公网安备 11010802027423号
京公网安备 11010802027423号