Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A stromal cell population that inhibits adipogenesis in mammalian fat depots
Nature ( IF 50.5 ) Pub Date : 2018-06-20 , DOI: 10.1038/s41586-018-0226-8 Petra C. Schwalie , Hua Dong , Magda Zachara , Julie Russeil , Daniel Alpern , Nassila Akchiche , Christian Caprara , Wenfei Sun , Kai-Uwe Schlaudraff , Gianni Soldati , Christian Wolfrum , Bart Deplancke
Nature ( IF 50.5 ) Pub Date : 2018-06-20 , DOI: 10.1038/s41586-018-0226-8 Petra C. Schwalie , Hua Dong , Magda Zachara , Julie Russeil , Daniel Alpern , Nassila Akchiche , Christian Caprara , Wenfei Sun , Kai-Uwe Schlaudraff , Gianni Soldati , Christian Wolfrum , Bart Deplancke
|
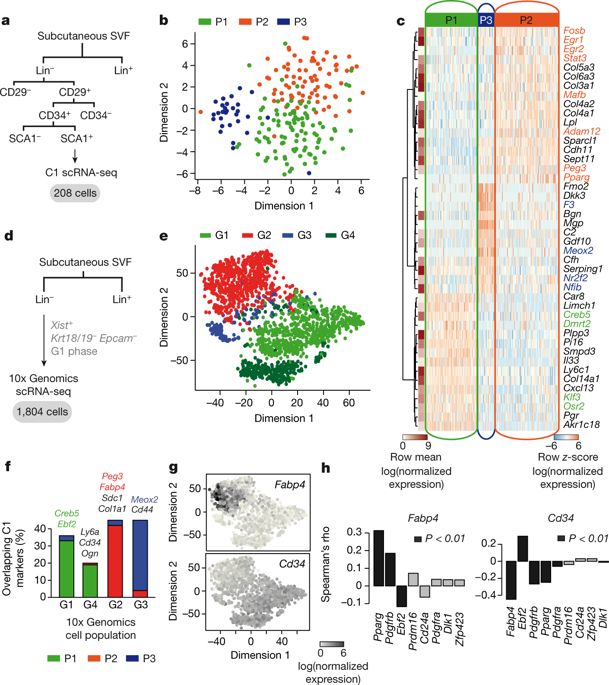
Adipocyte development and differentiation have an important role in the aetiology of obesity and its co-morbidities1,2. Although multiple studies have investigated the adipogenic stem and precursor cells that give rise to mature adipocytes3–14, our understanding of their in vivo origin and properties is incomplete2,15,16. This is partially due to the highly heterogeneous and unstructured nature of adipose tissue depots17, which has proven difficult to molecularly dissect using classical approaches such as fluorescence-activated cell sorting and Cre–lox lines based on candidate marker genes16,18. Here, using the resolving power of single-cell transcriptomics19 in a mouse model, we reveal distinct subpopulations of adipose stem and precursor cells in the stromal vascular fraction of subcutaneous adipose tissue. We identify one of these subpopulations as CD142+ adipogenesis-regulatory cells, which can suppress adipocyte formation in vivo and in vitro in a paracrine manner. We show that adipogenesis-regulatory cells are refractory to adipogenesis and that they are functionally conserved in humans. Our findings point to a potentially critical role for adipogenesis-regulatory cells in modulating adipose tissue plasticity, which is linked to metabolic control, differential insulin sensitivity and type 2 diabetes.Single-cell transcriptomics reveals that, in mice and humans, a population of cells in the stromal vascular fraction of adipose tissue regulates adipogenesis by suppressing adipocyte formation in a paracrine manner.
中文翻译:

抑制哺乳动物脂肪库中脂肪生成的基质细胞群
脂肪细胞的发育和分化在肥胖及其合并症的病因学中具有重要作用1,2。尽管多项研究调查了产生成熟脂肪细胞的成脂干细胞和前体细胞 3-14,但我们对它们的体内起源和特性的了解是不完整的 2,15,16。这部分是由于脂肪组织库的高度异质性和非结构化性质 17,已证明难以使用经典方法(如荧光激活细胞分选和基于候选标记基因的 Cre-lox 系 16,18 进行分子解剖)。在这里,我们在小鼠模型中使用单细胞转录组学的分辨能力,揭示了皮下脂肪组织的基质血管部分中脂肪干细胞和前体细胞的不同亚群。我们将这些亚群中的一个确定为 CD142+ 脂肪生成调节细胞,它可以以旁分泌的方式在体内和体外抑制脂肪细胞的形成。我们表明脂肪生成调节细胞对脂肪生成具有抵抗力,并且它们在人类中功能保守。我们的研究结果表明,脂肪生成调节细胞在调节脂肪组织可塑性方面具有潜在的关键作用,这与代谢控制、差异胰岛素敏感性和 2 型糖尿病有关。单细胞转录组学表明,在小鼠和人类中,一群细胞在脂肪组织的基质血管部分中,通过以旁分泌方式抑制脂肪细胞形成来调节脂肪生成。我们表明脂肪生成调节细胞对脂肪生成具有抵抗力,并且它们在人类中功能保守。我们的研究结果表明,脂肪生成调节细胞在调节脂肪组织可塑性方面具有潜在的关键作用,这与代谢控制、差异胰岛素敏感性和 2 型糖尿病有关。单细胞转录组学表明,在小鼠和人类中,一群细胞在脂肪组织的基质血管部分中,通过以旁分泌方式抑制脂肪细胞形成来调节脂肪生成。我们表明脂肪生成调节细胞对脂肪生成具有抵抗力,并且它们在人类中功能保守。我们的研究结果表明,脂肪生成调节细胞在调节脂肪组织可塑性方面具有潜在的关键作用,这与代谢控制、胰岛素敏感性差异和 2 型糖尿病有关。单细胞转录组学表明,在小鼠和人类中,一组细胞在脂肪组织的基质血管部分中,通过以旁分泌方式抑制脂肪细胞形成来调节脂肪生成。
更新日期:2018-06-20
中文翻译:

抑制哺乳动物脂肪库中脂肪生成的基质细胞群
脂肪细胞的发育和分化在肥胖及其合并症的病因学中具有重要作用1,2。尽管多项研究调查了产生成熟脂肪细胞的成脂干细胞和前体细胞 3-14,但我们对它们的体内起源和特性的了解是不完整的 2,15,16。这部分是由于脂肪组织库的高度异质性和非结构化性质 17,已证明难以使用经典方法(如荧光激活细胞分选和基于候选标记基因的 Cre-lox 系 16,18 进行分子解剖)。在这里,我们在小鼠模型中使用单细胞转录组学的分辨能力,揭示了皮下脂肪组织的基质血管部分中脂肪干细胞和前体细胞的不同亚群。我们将这些亚群中的一个确定为 CD142+ 脂肪生成调节细胞,它可以以旁分泌的方式在体内和体外抑制脂肪细胞的形成。我们表明脂肪生成调节细胞对脂肪生成具有抵抗力,并且它们在人类中功能保守。我们的研究结果表明,脂肪生成调节细胞在调节脂肪组织可塑性方面具有潜在的关键作用,这与代谢控制、差异胰岛素敏感性和 2 型糖尿病有关。单细胞转录组学表明,在小鼠和人类中,一群细胞在脂肪组织的基质血管部分中,通过以旁分泌方式抑制脂肪细胞形成来调节脂肪生成。我们表明脂肪生成调节细胞对脂肪生成具有抵抗力,并且它们在人类中功能保守。我们的研究结果表明,脂肪生成调节细胞在调节脂肪组织可塑性方面具有潜在的关键作用,这与代谢控制、差异胰岛素敏感性和 2 型糖尿病有关。单细胞转录组学表明,在小鼠和人类中,一群细胞在脂肪组织的基质血管部分中,通过以旁分泌方式抑制脂肪细胞形成来调节脂肪生成。我们表明脂肪生成调节细胞对脂肪生成具有抵抗力,并且它们在人类中功能保守。我们的研究结果表明,脂肪生成调节细胞在调节脂肪组织可塑性方面具有潜在的关键作用,这与代谢控制、胰岛素敏感性差异和 2 型糖尿病有关。单细胞转录组学表明,在小鼠和人类中,一组细胞在脂肪组织的基质血管部分中,通过以旁分泌方式抑制脂肪细胞形成来调节脂肪生成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号