Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis
Nature ( IF 64.8 ) Pub Date : 2018-06-20 , DOI: 10.1038/s41586-018-0234-8 Yufan Liang 1 , Xiaheng Zhang 1 , David W C MacMillan 1
Nature ( IF 64.8 ) Pub Date : 2018-06-20 , DOI: 10.1038/s41586-018-0234-8 Yufan Liang 1 , Xiaheng Zhang 1 , David W C MacMillan 1
Affiliation
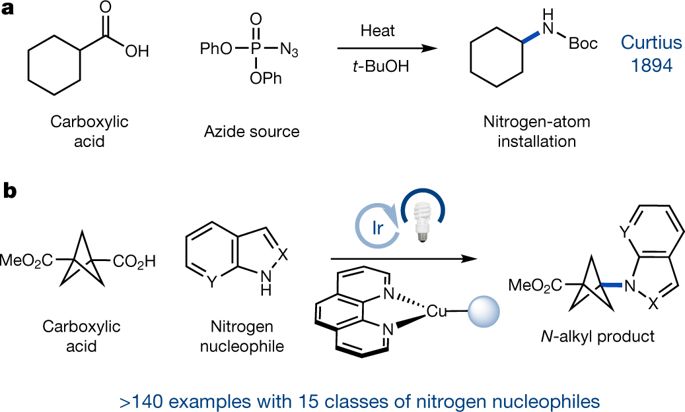
|
Over the past three decades, considerable progress has been made in the development of methods to construct sp2 carbon–nitrogen (C–N) bonds using palladium, copper or nickel catalysis1,2. However, the incorporation of alkyl substrates to form sp3 C–N bonds remains one of the major challenges in the field of cross-coupling chemistry. Here we demonstrate that the synergistic combination of copper catalysis and photoredox catalysis can provide a general platform from which to address this challenge. This cross-coupling system uses naturally abundant alkyl carboxylic acids and commercially available nitrogen nucleophiles as coupling partners. It is applicable to a wide variety of primary, secondary and tertiary alkyl carboxylic acids (through iodonium activation), as well as a vast array of nitrogen nucleophiles: nitrogen heterocycles, amides, sulfonamides and anilines can undergo C–N coupling to provide N-alkyl products in good to excellent efficiency, at room temperature and on short timescales (five minutes to one hour). We demonstrate that this C–N coupling protocol proceeds with high regioselectivity using substrates that contain several amine groups, and can also be applied to complex drug molecules, enabling the rapid construction of molecular complexity and the late-stage functionalization of bioactive pharmaceuticals.The synergistic combination of copper catalysis and photoredox catalysis forms sp3 C–N bonds in a rapid, room-temperature coupling protocol with high efficiencies and regioselectivities and a broad substrate scope.
中文翻译:

通过双铜和光氧化还原催化进行脱羧 sp3 C-N 偶联
在过去的三十年中,利用钯、铜或镍催化构建 sp2 碳氮 (C-N) 键的方法的开发取得了相当大的进展1,2。然而,结合烷基底物形成sp3 C-N键仍然是交叉偶联化学领域的主要挑战之一。在这里,我们证明铜催化和光氧化还原催化的协同组合可以提供一个通用平台来应对这一挑战。该交叉偶联系统使用天然丰富的烷基羧酸和市售的氮亲核试剂作为偶联伙伴。它适用于各种伯、仲和叔烷基羧酸(通过碘鎓活化),以及大量氮亲核试剂:氮杂环、酰胺、磺酰胺和苯胺可以进行 C-N 偶联,提供 N-在室温下和短时间内(五分钟到一小时),烷基产品的效率从良好到优异。我们证明,这种 C-N 偶联方案使用含有多个胺基的底物进行高区域选择性,并且还可以应用于复杂的药物分子,从而能够快速构建分子复杂性和生物活性药物的后期功能化。铜催化和光氧化还原催化的结合在快速、室温偶联方案中形成 sp3 C-N 键,具有高效率和区域选择性以及广泛的底物范围。
更新日期:2018-06-20
中文翻译:

通过双铜和光氧化还原催化进行脱羧 sp3 C-N 偶联
在过去的三十年中,利用钯、铜或镍催化构建 sp2 碳氮 (C-N) 键的方法的开发取得了相当大的进展1,2。然而,结合烷基底物形成sp3 C-N键仍然是交叉偶联化学领域的主要挑战之一。在这里,我们证明铜催化和光氧化还原催化的协同组合可以提供一个通用平台来应对这一挑战。该交叉偶联系统使用天然丰富的烷基羧酸和市售的氮亲核试剂作为偶联伙伴。它适用于各种伯、仲和叔烷基羧酸(通过碘鎓活化),以及大量氮亲核试剂:氮杂环、酰胺、磺酰胺和苯胺可以进行 C-N 偶联,提供 N-在室温下和短时间内(五分钟到一小时),烷基产品的效率从良好到优异。我们证明,这种 C-N 偶联方案使用含有多个胺基的底物进行高区域选择性,并且还可以应用于复杂的药物分子,从而能够快速构建分子复杂性和生物活性药物的后期功能化。铜催化和光氧化还原催化的结合在快速、室温偶联方案中形成 sp3 C-N 键,具有高效率和区域选择性以及广泛的底物范围。



























 京公网安备 11010802027423号
京公网安备 11010802027423号