Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Codon-specific translation reprogramming promotes resistance to targeted therapy
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0243-7 Francesca Rapino 1, 2 , Sylvain Delaunay 1, 2 , Florian Rambow 3, 4 , Zhaoli Zhou 1, 2 , Lars Tharun 5 , Pascal De Tullio 6 , Olga Sin 7, 8, 9 , Kateryna Shostak 2, 10 , Sebastian Schmitz 1, 2 , Jolanda Piepers 11 , Bart Ghesquière 12 , Latifa Karim 2, 13 , Benoit Charloteaux 2, 13 , Diane Jamart 1, 2 , Alexandra Florin 5 , Charles Lambert 2 , Andrée Rorive 14 , Guy Jerusalem 14 , Eleonora Leucci 3, 4 , Michael Dewaele 3, 4 , Marc Vooijs 11 , Sebastian A Leidel 7, 8, 9 , Michel Georges 2, 13 , Marianne Voz 2 , Bernard Peers 2 , Reinhard Büttner 5 , Jean-Christophe Marine 3, 4 , Alain Chariot 2, 10, 15 , Pierre Close 1, 2, 15
Nature ( IF 50.5 ) Pub Date : 2018-06-01 , DOI: 10.1038/s41586-018-0243-7 Francesca Rapino 1, 2 , Sylvain Delaunay 1, 2 , Florian Rambow 3, 4 , Zhaoli Zhou 1, 2 , Lars Tharun 5 , Pascal De Tullio 6 , Olga Sin 7, 8, 9 , Kateryna Shostak 2, 10 , Sebastian Schmitz 1, 2 , Jolanda Piepers 11 , Bart Ghesquière 12 , Latifa Karim 2, 13 , Benoit Charloteaux 2, 13 , Diane Jamart 1, 2 , Alexandra Florin 5 , Charles Lambert 2 , Andrée Rorive 14 , Guy Jerusalem 14 , Eleonora Leucci 3, 4 , Michael Dewaele 3, 4 , Marc Vooijs 11 , Sebastian A Leidel 7, 8, 9 , Michel Georges 2, 13 , Marianne Voz 2 , Bernard Peers 2 , Reinhard Büttner 5 , Jean-Christophe Marine 3, 4 , Alain Chariot 2, 10, 15 , Pierre Close 1, 2, 15
Affiliation
|
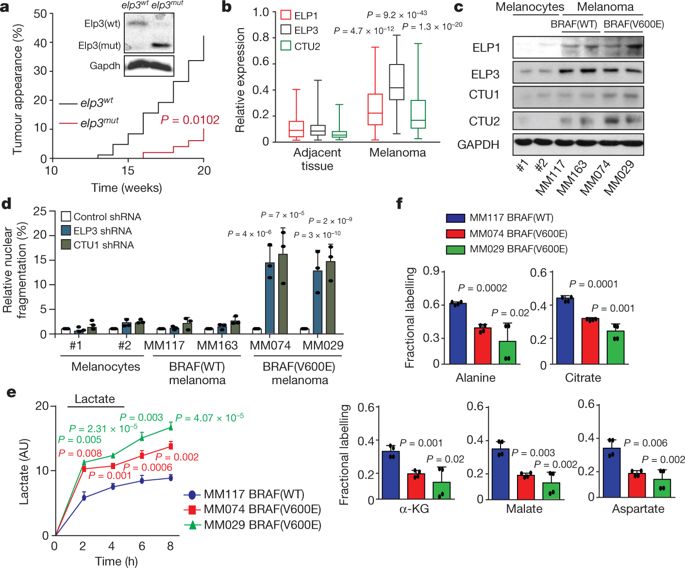
Reprogramming of mRNA translation has a key role in cancer development and drug resistance1. However, the molecular mechanisms that are involved in this process remain poorly understood. Wobble tRNA modifications are required for specific codon decoding during translation2,3. Here we show, in humans, that the enzymes that catalyse modifications of wobble uridine 34 (U34) tRNA (U34 enzymes) are key players of the protein synthesis rewiring that is induced by the transformation driven by the BRAFV600E oncogene and by resistance to targeted therapy in melanoma. We show that BRAFV600E-expressing melanoma cells are dependent on U34 enzymes for survival, and that concurrent inhibition of MAPK signalling and ELP3 or CTU1 and/or CTU2 synergizes to kill melanoma cells. Activation of the PI3K signalling pathway, one of the most common mechanisms of acquired resistance to MAPK therapeutic agents, markedly increases the expression of U34 enzymes. Mechanistically, U34 enzymes promote glycolysis in melanoma cells through the direct, codon-dependent, regulation of the translation of HIF1A mRNA and the maintenance of high levels of HIF1α protein. Therefore, the acquired resistance to anti-BRAF therapy is associated with high levels of U34 enzymes and HIF1α. Together, these results demonstrate that U34 enzymes promote the survival and resistance to therapy of melanoma cells by regulating specific mRNA translation.Enzymes that catalyse modifications of wobble uridine 34 tRNA are essential for the survival of melanoma cells that rely on HIF1α-dependent metabolism through codon-dependent regulation of the translation of HIF1A mRNA.
中文翻译:

密码子特异性翻译重编程促进对靶向治疗的抵抗
mRNA 翻译的重编程在癌症发展和耐药性中具有关键作用1。然而,参与这一过程的分子机制仍然知之甚少。翻译过程中特定密码子解码需要摆动 tRNA 修饰2,3。在这里,我们表明,在人类中,催化摆动尿苷 34 (U34) tRNA(U34 酶)修饰的酶是蛋白质合成重新布线的关键参与者,该重新布线是由 BRAFV600E 癌基因驱动的转化和对靶向治疗的抗性诱导的在黑色素瘤中。我们表明表达 BRAFV600E 的黑色素瘤细胞依赖 U34 酶才能存活,并且同时抑制 MAPK 信号和 ELP3 或 CTU1 和/或 CTU2 协同杀死黑色素瘤细胞。PI3K 信号通路的激活,对 MAPK 治疗剂产生抗药性的最常见机制之一,显着增加了 U34 酶的表达。从机制上讲,U34 酶通过直接的、密码子依赖的、调节 HIF1A mRNA 的翻译和维持高水平的 HIF1α 蛋白来促进黑色素瘤细胞中的糖酵解。因此,对抗 BRAF 治疗的获得性耐药与高水平的 U34 酶和 HIF1α 相关。总之,这些结果表明 U34 酶通过调节特定的 mRNA 翻译来促进黑色素瘤细胞的存活和对治疗的抵抗力。催化摆动尿苷 34 tRNA 修饰的酶对于依赖 HIF1α 依赖代谢的黑色素瘤细胞的存活至关重要。依赖于 HIF1A mRNA 翻译的调节。
更新日期:2018-06-01
中文翻译:

密码子特异性翻译重编程促进对靶向治疗的抵抗
mRNA 翻译的重编程在癌症发展和耐药性中具有关键作用1。然而,参与这一过程的分子机制仍然知之甚少。翻译过程中特定密码子解码需要摆动 tRNA 修饰2,3。在这里,我们表明,在人类中,催化摆动尿苷 34 (U34) tRNA(U34 酶)修饰的酶是蛋白质合成重新布线的关键参与者,该重新布线是由 BRAFV600E 癌基因驱动的转化和对靶向治疗的抗性诱导的在黑色素瘤中。我们表明表达 BRAFV600E 的黑色素瘤细胞依赖 U34 酶才能存活,并且同时抑制 MAPK 信号和 ELP3 或 CTU1 和/或 CTU2 协同杀死黑色素瘤细胞。PI3K 信号通路的激活,对 MAPK 治疗剂产生抗药性的最常见机制之一,显着增加了 U34 酶的表达。从机制上讲,U34 酶通过直接的、密码子依赖的、调节 HIF1A mRNA 的翻译和维持高水平的 HIF1α 蛋白来促进黑色素瘤细胞中的糖酵解。因此,对抗 BRAF 治疗的获得性耐药与高水平的 U34 酶和 HIF1α 相关。总之,这些结果表明 U34 酶通过调节特定的 mRNA 翻译来促进黑色素瘤细胞的存活和对治疗的抵抗力。催化摆动尿苷 34 tRNA 修饰的酶对于依赖 HIF1α 依赖代谢的黑色素瘤细胞的存活至关重要。依赖于 HIF1A mRNA 翻译的调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号