当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Air-Stable Direct Bandgap Perovskite Semiconductors: All-Inorganic Tin-Based Heteroleptic Halides AxSnClyIz (A = Cs, Rb)
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1021/acs.chemmater.8b02232 Jiangwei Li 1, 2 , Constantinos C. Stoumpos 2 , Giancarlo G. Trimarchi 2 , In Chung 2 , Lingling Mao 2 , Michelle Chen 2 , Michael R. Wasielewski 2 , Liduo Wang 1 , Mercouri G. Kanatzidis 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1021/acs.chemmater.8b02232 Jiangwei Li 1, 2 , Constantinos C. Stoumpos 2 , Giancarlo G. Trimarchi 2 , In Chung 2 , Lingling Mao 2 , Michelle Chen 2 , Michael R. Wasielewski 2 , Liduo Wang 1 , Mercouri G. Kanatzidis 2
Affiliation
|
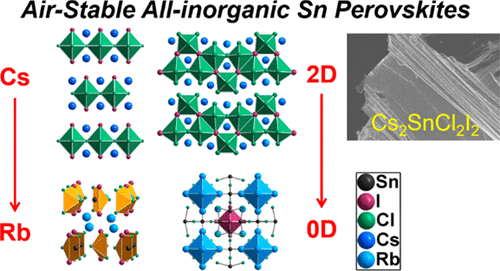
Semiconducting halide perovskites are a class of materials with exciting photoelectronic properties. Compared to the widely studied hybrid organic–inorganic perovskites, the all-inorganic derivatives are less well understood even as they promise high inherent stability. Currently, such materials are limited due to the fact that there is a very narrow choice of inorganic cations that can stabilize the desirable perovskite structure. Herein we report on the synthesis and characterization of novel all-inorganic tin-based perovskites and perovskitoids that can be stabilized by the heteroleptic coordination of chloride and iodide anions, Cs2SnCl2I2 (1) and Cs2.38Rb1.62Sn3Cl8I2 (2), consist of two-dimensional (2D) layers of [SnCl4I2]4– octahedra with different connectivity modes. Compound 1 is an n = 1 Ruddlesden–Popper type perovskite adopting the tetragonal archetype structure (I4/mmm space group; a = 5.5905(3) Å, c = 18.8982(13) Å), while compound 2 crystallizes as an orthorhombic modification (Cmcm space group; a = 5.6730(11) Å, b = 25.973(5) Å, c = 16.587(3) Å) with corrugated layers. The crystal chemistry changes drastically when Cs+ is replaced by the smaller Rb+ cation which leads to the isolation of the low dimensional compounds Rb3SnCl3I2 (3a), Rb3SnCl2.33I2.67 (3b) and Rb7Sn4.25Cl12I3.5 (4), thus illustrating the importance of the A-cation size in the formation of perovskites. The 2D perovskites show wide band gaps and relatively large resistivities, associated with their chemical stability against the oxidation of Sn2+. The chemical stability is coupled with remarkable electronic properties that derive from the perovskite structure. DFT calculations suggest that both compounds are direct band gap semiconductors with large bandwidths, consistently with the experimentally determined band gaps of Eg = 2.62 and 2.81 eV for 1 and 2, respectively. The combination of stability and favorable electronic structure in heteroleptic-halide perovskites presents a new direction toward the realization of functional devices made exclusively from inorganic perovskites.
中文翻译:

空气稳定的直接带隙钙钛矿半导体:全无机锡基杂多卤化物A x SnCl y I z(A = Cs,Rb)
半导体卤化物钙钛矿是一类具有令人兴奋的光电特性的材料。与广泛研究的杂化有机-无机钙钛矿杂化相比,尽管全无机衍生物具有很高的固有稳定性,但人们对其了解较少。当前,由于可以稳定所需的钙钛矿结构的无机阳离子的选择非常狭窄的事实,限制了这种材料。在这里,我们报告了新型的全无机锡基钙钛矿和钙钛矿类化合物的合成和表征,它们可以通过氯和碘化物阴离子,Cs 2 SnCl 2 I 2(1)和Cs 2.38 Rb 1.62 Sn 3的杂合配位作用来稳定。Cl 8 I 2(2)由具有不同连接模式的[SnCl 4 I 2 ] 4–八面体的二维(2D)层组成。化合物1是n = 1的Ruddlesden-Popper型钙钛矿,采用四方原型结构(I 4 / mmm空间群; a = 5.5905(3)Å,c = 18.8982(13)Å),而化合物2结晶为正交晶型。 (Cmcm空间组; a = 5.6730(11)Å,b = 25.973(5)Å,c = 16.587(3)Å)带波纹层。当Cs +被较小的Rb取代时,晶体化学急剧变化+阳离子导致低维化合物Rb 3 SnCl 3 I 2(3a),Rb 3 SnCl 2.33 I 2.67(3b)和Rb 7 Sn 4.25 Cl 12 I 3.5(4)的分离,因此说明了钙钛矿中形成A-阳离子的大小。二维钙钛矿显示出宽带隙和相对较大的电阻率,这与其抗Sn 2+氧化的化学稳定性有关。。化学稳定性与源自钙钛矿结构的出色电子性能相结合。DFT计算表明,这两种化合物都是具有大带宽的直接带隙半导体,与通过实验确定的1和2的E g分别为2.62和2.81 eV的带隙一致。杂卤化物钙钛矿中稳定性和良好的电子结构的结合为实现仅由无机钙钛矿制成的功能器件提供了新的方向。
更新日期:2018-06-20
中文翻译:

空气稳定的直接带隙钙钛矿半导体:全无机锡基杂多卤化物A x SnCl y I z(A = Cs,Rb)
半导体卤化物钙钛矿是一类具有令人兴奋的光电特性的材料。与广泛研究的杂化有机-无机钙钛矿杂化相比,尽管全无机衍生物具有很高的固有稳定性,但人们对其了解较少。当前,由于可以稳定所需的钙钛矿结构的无机阳离子的选择非常狭窄的事实,限制了这种材料。在这里,我们报告了新型的全无机锡基钙钛矿和钙钛矿类化合物的合成和表征,它们可以通过氯和碘化物阴离子,Cs 2 SnCl 2 I 2(1)和Cs 2.38 Rb 1.62 Sn 3的杂合配位作用来稳定。Cl 8 I 2(2)由具有不同连接模式的[SnCl 4 I 2 ] 4–八面体的二维(2D)层组成。化合物1是n = 1的Ruddlesden-Popper型钙钛矿,采用四方原型结构(I 4 / mmm空间群; a = 5.5905(3)Å,c = 18.8982(13)Å),而化合物2结晶为正交晶型。 (Cmcm空间组; a = 5.6730(11)Å,b = 25.973(5)Å,c = 16.587(3)Å)带波纹层。当Cs +被较小的Rb取代时,晶体化学急剧变化+阳离子导致低维化合物Rb 3 SnCl 3 I 2(3a),Rb 3 SnCl 2.33 I 2.67(3b)和Rb 7 Sn 4.25 Cl 12 I 3.5(4)的分离,因此说明了钙钛矿中形成A-阳离子的大小。二维钙钛矿显示出宽带隙和相对较大的电阻率,这与其抗Sn 2+氧化的化学稳定性有关。。化学稳定性与源自钙钛矿结构的出色电子性能相结合。DFT计算表明,这两种化合物都是具有大带宽的直接带隙半导体,与通过实验确定的1和2的E g分别为2.62和2.81 eV的带隙一致。杂卤化物钙钛矿中稳定性和良好的电子结构的结合为实现仅由无机钙钛矿制成的功能器件提供了新的方向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号