当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
EGF Regulates the Interaction of Tks4 with Src through Its SH2 and SH3 Domains
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acs.biochem.8b00084 Metta Dülk 1 , Bálint Szeder 1 , Gábor Glatz 2 , Balázs L. Merő 1 , Kitti Koprivanacz 1 , Gyöngyi Kudlik 1 , Virág Vas 1 , Szabolcs Sipeki 3 , Anna Cserkaszky 1 , László Radnai 1 , László Buday 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acs.biochem.8b00084 Metta Dülk 1 , Bálint Szeder 1 , Gábor Glatz 2 , Balázs L. Merő 1 , Kitti Koprivanacz 1 , Gyöngyi Kudlik 1 , Virág Vas 1 , Szabolcs Sipeki 3 , Anna Cserkaszky 1 , László Radnai 1 , László Buday 1, 3
Affiliation
|
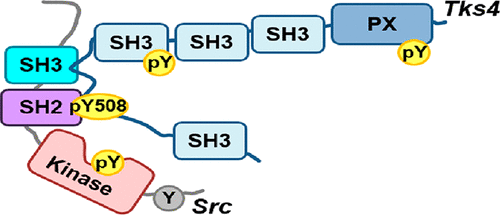
The nonreceptor tyrosine kinase Src is a central component of the epidermal growth factor (EGF) signaling pathway. Our group recently showed that the Frank-ter Haar syndrome protein Tks4 (tyrosine kinase substrate with four Src homology 3 domains) is also involved in EGF signaling. Here we demonstrate that Tks4 and Src bind directly to each other and elucidate the details of the molecular mechanism of this complex formation. Results of GST pull-down and fluorescence polarization assays show that both a proline-rich SH3 binding motif (PSRPLPDAP, residues 466–474) and an adjacent phosphotyrosine-containing SH2 binding motif (pYEEI, residues 508–511) in Tks4 are responsible for Src binding. These motifs interact with the SH3 and SH2 domains of Src, respectively, leading to a synergistic enhancement of binding strength and a highly stable, “bidentate”-type of interaction. In agreement with these results, we found that the association of Src with Tks4 is permanent and the complex lasts at least 3 h in living cells. We conclude that the interaction of Tks4 with Src may result in the long term stabilization of the kinase in its active conformation, leading to prolonged Src activity following EGF stimulation.
中文翻译:

EGF通过其SH2和SH3结构域调节Tks4与Src的相互作用
非受体酪氨酸激酶Src是表皮生长因子(EGF)信号传导途径的重要组成部分。我们的小组最近表明,Frank-ter Haar综合征蛋白Tks4(具有四个Src同源性3结构域的酪氨酸激酶底物)也参与EGF信号传导。在这里,我们证明Tks4和Src直接彼此结合,并阐明这种复合物形成的分子机制的细节。GST下拉和荧光偏振测定的结果表明,Tks4中富含脯氨酸的SH3结合基序(PSRPLPDAP,残基466–474)和相邻的含磷酸酪氨酸的SH2结合基序(pYEEI,残基508–511)均与Tks4的产生有关Src绑定。这些基序分别与Src的SH3和SH2域相互作用,从而导致结合强度的协同增强和高度稳定,“双向”类型的交互。与这些结果一致,我们发现Src与Tks4的结合是永久的,并且该复合物在活细胞中持续至少3 h。我们得出结论,Tks4与Src的相互作用可能会导致激酶处于其主动构象的长期稳定状态,从而导致EGF刺激后Src活性延长。
更新日期:2018-06-21
中文翻译:

EGF通过其SH2和SH3结构域调节Tks4与Src的相互作用
非受体酪氨酸激酶Src是表皮生长因子(EGF)信号传导途径的重要组成部分。我们的小组最近表明,Frank-ter Haar综合征蛋白Tks4(具有四个Src同源性3结构域的酪氨酸激酶底物)也参与EGF信号传导。在这里,我们证明Tks4和Src直接彼此结合,并阐明这种复合物形成的分子机制的细节。GST下拉和荧光偏振测定的结果表明,Tks4中富含脯氨酸的SH3结合基序(PSRPLPDAP,残基466–474)和相邻的含磷酸酪氨酸的SH2结合基序(pYEEI,残基508–511)均与Tks4的产生有关Src绑定。这些基序分别与Src的SH3和SH2域相互作用,从而导致结合强度的协同增强和高度稳定,“双向”类型的交互。与这些结果一致,我们发现Src与Tks4的结合是永久的,并且该复合物在活细胞中持续至少3 h。我们得出结论,Tks4与Src的相互作用可能会导致激酶处于其主动构象的长期稳定状态,从而导致EGF刺激后Src活性延长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号