当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Primary Deuterium Kinetic Isotope Effects: A Probe for the Origin of the Rate Acceleration for Hydride Transfer Catalyzed by Glycerol-3-Phosphate Dehydrogenase.
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.biochem.8b00536 Archie C Reyes 1 , Tina L Amyes 1 , John P Richard 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-07-10 , DOI: 10.1021/acs.biochem.8b00536 Archie C Reyes 1 , Tina L Amyes 1 , John P Richard 1
Affiliation
|
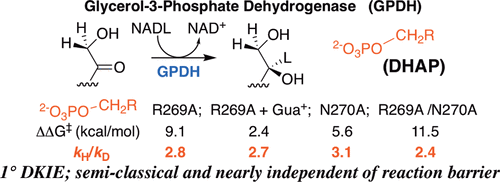
Large primary deuterium kinetic isotope effects (1° DKIEs) on enzyme-catalyzed hydride transfer may be observed when the transferred hydride tunnels through the energy barrier. The following 1° DKIEs on kcat/ Km and relative reaction driving force are reported for wild-type and mutant glycerol-3-phosphate dehydrogenase (GPDH)-catalyzed reactions of NADL (L = H, D): wild-type GPDH, ΔΔ G⧧ = 0 kcal/mol, 1° DKIE = 1.5; N270A, 5.6 kcal/mol, 3.1; R269A, 9.1 kcal/mol, 2.8; R269A + 1.0 M guanidine, 2.4 kcal/mol, 2.7; R269A/N270A, 11.5 kcal/mol, 2.4. Similar 1° DKIEs were observed on kcat. The narrow range of 1° DKIEs (2.4-3.1) observed for a 9.1 kcal/mol change in reaction driving force provides strong evidence that these are intrinsic 1° DKIEs on rate-determining hydride transfer. Evidence is presented that the intrinsic DKIE on wild-type GPDH-catalyzed reduction of DHAP lies in this range. A similar range of 1° DKIEs (2.4-2.9) on ( kcat/ KGA, M-1 s-1) was reported for dianion-activated hydride transfer from NADL to glycolaldehyde (GA) [Reyes, A. C.; Amyes, T. L.; Richard, J. P. J. Am. Chem. Soc. 2016, 138, 14526-14529]. These 1° DKIEs are much smaller than those observed for enzyme-catalyzed hydrogen transfer that occurs mainly by quantum mechanical tunneling. These results support the conclusion that the rate acceleration for GPDH-catalyzed reactions is due to the stabilization of the transition state for hydride transfer by interactions with the protein catalyst. The small 1° DKIEs reported for mutant GPDH-catalyzed and for wild-type dianion-activated reactions are inconsistent with a model where the dianion binding energy is utilized in the stabilization of a tunneling ready state.
中文翻译:

初级氘动力学同位素效应:对由甘油-3-磷酸脱氢酶催化的氢化物转移速率加速起源的探索。
当转移的氢化物穿过能垒时,可以观察到对酶催化的氢化物转移的大的初级氘动力学同位素效应 (1° DKIE)。对于野生型和突变型 3-磷酸甘油脱氢酶 (GPDH) 催化的 NADL (L = H, D) 反应,报告了 kcat/Km 上的以下 1° DKIE 和相对反应驱动力:野生型 GPDH,ΔΔ G⧧ = 0 kcal/mol,1° DKIE = 1.5;N270A,5.6 kcal/mol,3.1;R269A,9.1 kcal/mol,2.8;R269A + 1.0 M 胍,2.4 kcal/mol,2.7;R269A/N270A,11.5 kcal/mol,2.4。在 kcat 上观察到类似的 1° DKIE。在反应驱动力发生 9.1 kcal/mol 变化时观察到的 1° DKIE (2.4-3.1) 的窄范围提供了强有力的证据,表明这些是决定氢化物转移速率的固有 1° DKIE。有证据表明,野生型 GPDH 催化的 DHAP 还原的内在 DKIE 位于此范围内。据报道,二价阴离子激活的氢化物从 NADL 转移到乙醇醛 (GA) [Reyes, AC; 艾米斯,TL;理查德,JPJ 上午。化学 社会。2016, 138, 14526-14529]。这些 1° DKIE 比观察到的主要通过量子力学隧道效应发生的酶催化氢转移的 DKIE 小得多。这些结果支持这样的结论,即 GPDH 催化反应的速率加速是由于通过与蛋白质催化剂的相互作用稳定了氢化物转移的过渡态。
更新日期:2018-06-21
中文翻译:

初级氘动力学同位素效应:对由甘油-3-磷酸脱氢酶催化的氢化物转移速率加速起源的探索。
当转移的氢化物穿过能垒时,可以观察到对酶催化的氢化物转移的大的初级氘动力学同位素效应 (1° DKIE)。对于野生型和突变型 3-磷酸甘油脱氢酶 (GPDH) 催化的 NADL (L = H, D) 反应,报告了 kcat/Km 上的以下 1° DKIE 和相对反应驱动力:野生型 GPDH,ΔΔ G⧧ = 0 kcal/mol,1° DKIE = 1.5;N270A,5.6 kcal/mol,3.1;R269A,9.1 kcal/mol,2.8;R269A + 1.0 M 胍,2.4 kcal/mol,2.7;R269A/N270A,11.5 kcal/mol,2.4。在 kcat 上观察到类似的 1° DKIE。在反应驱动力发生 9.1 kcal/mol 变化时观察到的 1° DKIE (2.4-3.1) 的窄范围提供了强有力的证据,表明这些是决定氢化物转移速率的固有 1° DKIE。有证据表明,野生型 GPDH 催化的 DHAP 还原的内在 DKIE 位于此范围内。据报道,二价阴离子激活的氢化物从 NADL 转移到乙醇醛 (GA) [Reyes, AC; 艾米斯,TL;理查德,JPJ 上午。化学 社会。2016, 138, 14526-14529]。这些 1° DKIE 比观察到的主要通过量子力学隧道效应发生的酶催化氢转移的 DKIE 小得多。这些结果支持这样的结论,即 GPDH 催化反应的速率加速是由于通过与蛋白质催化剂的相互作用稳定了氢化物转移的过渡态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号