当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methinylogation Approach in Chiral Pharmacophore Design: from Alkynyl‐ to Allenyl‐carbinol Warheads against Tumor Cells
ChemMedChem ( IF 3.6 ) Pub Date : 2018-07-23 , DOI: 10.1002/cmdc.201800284 Dymytrii Listunov 1 , Etienne Joly 2 , Carine Duhayon 1 , Nathalie Saffon-Merceron 3 , Isabelle Fabing 4 , Yves Génisson 4 , Valérie Maraval 1 , Remi Chauvin 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-07-23 , DOI: 10.1002/cmdc.201800284 Dymytrii Listunov 1 , Etienne Joly 2 , Carine Duhayon 1 , Nathalie Saffon-Merceron 3 , Isabelle Fabing 4 , Yves Génisson 4 , Valérie Maraval 1 , Remi Chauvin 1
Affiliation
|
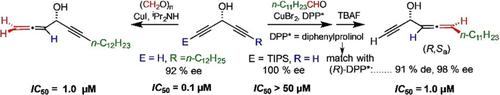
Extension of a structure–activity relationship study of the antitumor cytotoxicity of lipidic dialkynylcarbinols (DACs) is envisaged by formal methinylogation of one of the ethyndiyl moieties of the DAC warhead into the corresponding allenylalkynylcarbinol (AllAC) counterpart. External AllACs were directly obtained by methinylation of the parent DACs with formaldehyde in either the racemic or scalemic series. Isomers containing external progargyl and propynyl motifs were also prepared. Internal AllACs were obtained as racemic statistical mixtures of stereoisomers in two steps from the key C5‐DAC rac‐TIPS‐C≡C‐CH(OH)‐C≡CH and aldehydes. Kinetic resolution of the (S)‐C5‐DAC in 97 % ee and (R)‐C5‐DAC in 99 % ee was achieved by sequential lipase‐mediated acetylation/hydrolysis using the Candida antartica lipase (Novozyme 435). The four internal AllAC stereoisomers were prepared by asymmetric methinylation with (R)‐ or (S)‐diphenylprolinol as chiral auxiliary. Cytotoxicity assays on HCT116 cancer cells showed that the most active (eutomeric) external or internal AllAC exhibits an S configuration, a fatty chain length of n=12, and a 50 % inhibitory concentration IC50≈1.0 μm.
中文翻译:

手性药理团设计中的甲基化方法:从炔基到异戊二烯醛战斗部对抗肿瘤细胞
通过将DAC战斗部的乙炔基部分之一正式甲基化为相应的烯基炔基甲醇(AllAC)对应物,可以构想对脂质二炔基甲醇(DAC)的抗肿瘤细胞毒性的结构-活性关系研究的扩展。外消旋的AllACs是通过外消旋或水垢系列中的母体DAC与甲醛进行甲酰化直接获得的。还制备了含有外部炔丙基和丙炔基的异构体。从关键的C 5 -DAC rac -TIPS-C≡C-CH(OH)-C≡CH和醛类分两步获得内部AllAC作为立体异构体的外消旋统计混合物。(S)‐C 5‐ DAC在97% ee和(R中的动力学分辨率)-C 5 -DAC的99% ee是通过使用南极假丝酵母脂肪酶(Novozyme 435)进行的顺序脂肪酶介导的乙酰化/水解来实现的。通过(R)-或(S)-二苯基脯氨醇作为手性助剂的不对称甲基化反应制备了四种内部的AllAC立体异构体。上HCT116癌细胞的细胞毒性实验表明,最活跃的(eutomeric)的外部或内部AllAC呈现小号配置,脂肪链的长度Ñ = 12,和50%的抑制浓度IC 50 ≈1.0μ米。
更新日期:2018-07-23
中文翻译:

手性药理团设计中的甲基化方法:从炔基到异戊二烯醛战斗部对抗肿瘤细胞
通过将DAC战斗部的乙炔基部分之一正式甲基化为相应的烯基炔基甲醇(AllAC)对应物,可以构想对脂质二炔基甲醇(DAC)的抗肿瘤细胞毒性的结构-活性关系研究的扩展。外消旋的AllACs是通过外消旋或水垢系列中的母体DAC与甲醛进行甲酰化直接获得的。还制备了含有外部炔丙基和丙炔基的异构体。从关键的C 5 -DAC rac -TIPS-C≡C-CH(OH)-C≡CH和醛类分两步获得内部AllAC作为立体异构体的外消旋统计混合物。(S)‐C 5‐ DAC在97% ee和(R中的动力学分辨率)-C 5 -DAC的99% ee是通过使用南极假丝酵母脂肪酶(Novozyme 435)进行的顺序脂肪酶介导的乙酰化/水解来实现的。通过(R)-或(S)-二苯基脯氨醇作为手性助剂的不对称甲基化反应制备了四种内部的AllAC立体异构体。上HCT116癌细胞的细胞毒性实验表明,最活跃的(eutomeric)的外部或内部AllAC呈现小号配置,脂肪链的长度Ñ = 12,和50%的抑制浓度IC 50 ≈1.0μ米。











































 京公网安备 11010802027423号
京公网安备 11010802027423号