当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Stereoselective Michael Addition of N- and S- Nucleophiles to a d-Erythrosyl 1,5-Lactone Derivative. Experimental and Theoretical Studies Devoted to the Synthesis of 2,6-Dideoxy-4-functionalized-d-ribono-hexono-1,4-lactone
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1021/acs.joc.8b00769 Juliana F. Rocha 1 , David S. Freitas 2 , Jennifer Noro 2 , Carla S. Silva Teixeira 1 , Cristina E. A. Sousa 2 , Maria J. Alves 2 , Nuno M. F. S. A. Cerqueira 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-06-20 00:00:00 , DOI: 10.1021/acs.joc.8b00769 Juliana F. Rocha 1 , David S. Freitas 2 , Jennifer Noro 2 , Carla S. Silva Teixeira 1 , Cristina E. A. Sousa 2 , Maria J. Alves 2 , Nuno M. F. S. A. Cerqueira 1
Affiliation
|
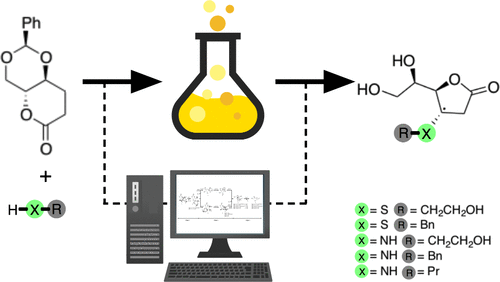
The synthesis of a 1,5-lactone 2,4-O-alkylidene-d-erythrose derivative was found to be a highly stereoselective template in Michael addition trough the reaction of a d-erythrosyl 1,5-lactone derivative with nitrogen and sulfur nucleophiles. The sulfur adducts formed are 1 (d-erythrose derivative):1 (nucleophile), and the nitrogen adducts are 1:2. Both were then treated under HCl to give 2,6-dideoxy-4-functionalized-d-ribono-hexono-1,4-lactone by a reaction cascade in high overall yield. Reaction’s scale up even improves the yield. The theoretical and computational results clearly explain the origin of the stereoselectivity, and the energetic course of reactions starting with nitrogen and sulfide nucleophiles. Considering that the 1,4-lactones obtained in this work offer a new molecular scaffold for organic synthesis, these new results provide a solid theoretical platform that can be used to speed up synthesis of other derivatives in a stereo- and regioselective way.
中文翻译:

N-和S-亲核试剂与d-赤藓糖基1,5-内酯衍生物的总立体选择性Michael加成。致力于2,6-二脱氧-4-官能化-d-核糖-己酮-1,4-内酯的合成的实验和理论研究
的合成-1,5-内酯-2,4- ö -alkylidene- d -erythrose衍生物被发现是在Michael加成槽a的反应高度立体选择性模板d -erythrosyl -1,5-内酯衍生物与氮和硫亲核试剂。形成的硫加合物为1(d-赤藓糖衍生物):1(亲核试剂),氮加合物为1:2。两者都然后加入HCl下处理,得到2,6-二脱氧-4- functionalized- d -核糖酸-hexono-1,4-内酯通过反应级联以高总收率获得。反应的放大甚至可以提高产量。理论和计算结果清楚地说明了立体选择性的起源,以及从氮和硫化物亲核试剂开始的高能反应过程。考虑到这项工作中获得的1,4-内酯为有机合成提供了新的分子支架,这些新结果提供了坚实的理论平台,可用于以立体和区域选择性方式加快其他衍生物的合成。
更新日期:2018-06-20
中文翻译:

N-和S-亲核试剂与d-赤藓糖基1,5-内酯衍生物的总立体选择性Michael加成。致力于2,6-二脱氧-4-官能化-d-核糖-己酮-1,4-内酯的合成的实验和理论研究
的合成-1,5-内酯-2,4- ö -alkylidene- d -erythrose衍生物被发现是在Michael加成槽a的反应高度立体选择性模板d -erythrosyl -1,5-内酯衍生物与氮和硫亲核试剂。形成的硫加合物为1(d-赤藓糖衍生物):1(亲核试剂),氮加合物为1:2。两者都然后加入HCl下处理,得到2,6-二脱氧-4- functionalized- d -核糖酸-hexono-1,4-内酯通过反应级联以高总收率获得。反应的放大甚至可以提高产量。理论和计算结果清楚地说明了立体选择性的起源,以及从氮和硫化物亲核试剂开始的高能反应过程。考虑到这项工作中获得的1,4-内酯为有机合成提供了新的分子支架,这些新结果提供了坚实的理论平台,可用于以立体和区域选择性方式加快其他衍生物的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号