Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms of mercury transformation over α-Fe 2 O 3 (0 0 1) in the presence of HCl and/or H 2 S
Fuel ( IF 6.7 ) Pub Date : 2018-12-01 , DOI: 10.1016/j.fuel.2018.06.065 Yu Chen , Xin Guo , Fan Wu , Yu Huang , Zhanchi Yin
Fuel ( IF 6.7 ) Pub Date : 2018-12-01 , DOI: 10.1016/j.fuel.2018.06.065 Yu Chen , Xin Guo , Fan Wu , Yu Huang , Zhanchi Yin
|
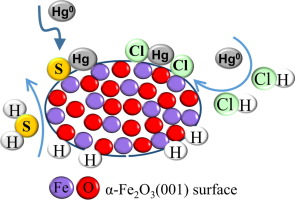
Abstract Hematite (α-Fe 2 O 3 ) has been proved to be a promising sorbent for mercury removal in various situations. The mercury removal performance of α-Fe 2 O 3 was investigated using a simulated coal gasification syngas containing Hg 0 , N 2 , HCl, and/or H 2 S, a series of experiments were designed with HCl and H 2 S separately and both employed. For a better understanding in the interaction between HCl and H 2 S in mercury removal, the differences in mercury oxidation were demonstrated on the basis of density functional theory calculations. The rate-limiting step in HgCl 2 formation is Hg 0 → HgCl with the energy barrier of 139.47 kJ/mol, but for H 2 S in mercury oxidation, the generation of active sulfur species is the key step and the rate-limiting step is detachment of second H with an energy barrier of 57.53 kJ/mol. Experimental results point that HCl has inhibition on H 2 S for mercury removal, it may be ascribed to the competition of active Fe sites on Fe 2 O 3 . Conversely, HCl contributes to the mercury oxidation at high temperature while H 2 S has a limit effect at elevated temperature.
中文翻译:

在 HCl 和/或 H 2 S 存在下,α-Fe 2 O 3 (0 0 1) 上汞转化的机理
摘要 赤铁矿 (α-Fe 2 O 3 ) 已被证明是一种在各种情况下脱汞的有前途的吸附剂。使用含Hg 0 、N 2 、HCl 和/或H 2 S 的模拟煤气化合成气研究α-Fe 2 O 3 的除汞性能,分别设计了一系列实验,分别使用HCl 和H 2 S受雇。为了更好地理解 HCl 和 H 2 S 在除汞过程中的相互作用,在密度泛函理论计算的基础上证明了汞氧化的差异。HgCl 2 形成的限速步骤为Hg 0 → HgCl,能垒为139.47 kJ/mol,但对于汞氧化中的H 2 S,活性硫物种的生成是关键步骤,限速步骤为第二个 H 的分离,能量势垒为 57.53 kJ/mol。实验结果表明,HCl 对 H 2 S 的除汞有抑制作用,这可能是由于活性 Fe 位点对 Fe 2 O 3 的竞争。相反,HCl 在高温下有助于汞氧化,而 H 2 S 在高温下具有限制作用。
更新日期:2018-12-01
中文翻译:

在 HCl 和/或 H 2 S 存在下,α-Fe 2 O 3 (0 0 1) 上汞转化的机理
摘要 赤铁矿 (α-Fe 2 O 3 ) 已被证明是一种在各种情况下脱汞的有前途的吸附剂。使用含Hg 0 、N 2 、HCl 和/或H 2 S 的模拟煤气化合成气研究α-Fe 2 O 3 的除汞性能,分别设计了一系列实验,分别使用HCl 和H 2 S受雇。为了更好地理解 HCl 和 H 2 S 在除汞过程中的相互作用,在密度泛函理论计算的基础上证明了汞氧化的差异。HgCl 2 形成的限速步骤为Hg 0 → HgCl,能垒为139.47 kJ/mol,但对于汞氧化中的H 2 S,活性硫物种的生成是关键步骤,限速步骤为第二个 H 的分离,能量势垒为 57.53 kJ/mol。实验结果表明,HCl 对 H 2 S 的除汞有抑制作用,这可能是由于活性 Fe 位点对 Fe 2 O 3 的竞争。相反,HCl 在高温下有助于汞氧化,而 H 2 S 在高温下具有限制作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号