Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spin-Crossover Temperature Predictable from DFT Calculation for Iron(II) Complexes with 4-Substituted Pybox and Related Heteroaromatic Ligands
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acsomega.8b01095 Akifumi Kimura 1 , Takayuki Ishida 1
ACS Omega ( IF 3.7 ) Pub Date : 2018-06-21 00:00:00 , DOI: 10.1021/acsomega.8b01095 Akifumi Kimura 1 , Takayuki Ishida 1
Affiliation
|
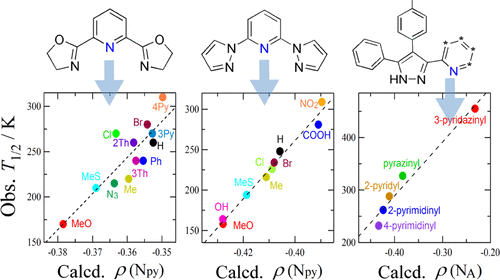
Spin-crossover (SCO) is a reversible transition between low and high spin states by external stimuli such as heat. The SCO behavior and transition temperature (T1/2) of a series of [FeII(X-pybox)2](ClO4)2 were studied to establish a methodology for ligand-field engineering, where X-pybox stands for 2,6-bis(oxazolin-2-yl)pyridine substituted with X at the 4-position of the pyridine ring. We utilized X = MeO, Me, 3-thienyl, Ph, H, MeS, 2-thienyl, N3, Cl, Br, 3-pyridyl, and 4-pyridyl. The solution susceptometry on five new derivatives with X = Me, 2-thienyl, N3, Br, and 3-pyridyl was performed in acetone, giving the SCO temperatures of 220, 260, 215, 280, and 270 K, respectively. The density-functional-theory molecular orbital (MO) calculation was performed on the ligands with geometry optimization. The atomic charge on the pyridine nitrogen atom [ρ(Npy)] was extracted from the natural orbital population analysis. Positive correlation appeared in the T1/2 versus ρ(Npy) plot with R2 = 0.734, being consistent with the analysis using the Hammett substituent constants (σp and σp+). This finding well agrees with the mechanism proposed: the rich electron density lifts the t2g energy level through the dπ–pπ interaction, resulting in a narrow t2g–eg energy gap and favoring the high-spin state and low T1/2. The MO method was successfully applied to the known SCO-active iron(II) compounds involving 4-substituted 2,6-bis(pyrazol-1-yl)pyridines. A distinct positive correlation appeared in the T1/2 versus ρ(Npy) plot. The comparison of correlation coefficients indicates that ρ(Npy) is a more reliable parameter than σp or σp+ to predict a shift of T1/2. Furthermore, this method can be more generalized by application to another known SCO family having 3-azinyl-4-p-tolyl-5-phenyl-1,2,4-triazole ligand series, where azinyl stands for a 2-azaaromatic ring. A good linear correlation was found in the T1/2 versus ρ(NA) plot (NA is the ligating nitrogen atom in the azaaromatic ring). Finally, we will state a reason why the present treatment is competent to predict the SCO equilibrium position only by consideration on the electronic perturbation.
中文翻译:

从DFT计算可预测具有4个取代的Pybox和相关杂芳族配体的铁(II)配合物的自旋交叉温度
自旋交叉(SCO)是在低自旋状态和高自旋状态之间通过外部刺激(例如热)的可逆转变。研究了一系列[Fe II(X-pybox)2 ](ClO 4)2的SCO行为和转变温度(T 1/2),建立了配体场工程学方法,其中X-pybox代表2在吡啶环的4-位被X取代的,6-双(恶唑啉-2-基)吡啶。我们利用X = MeO,Me,3-噻吩基,Ph,H,MeS,2-噻吩基,N 3,Cl,Br,3-吡啶基和4-吡啶基。X = Me,2-噻吩基,N 3的五种新衍生物的溶液磁化法,Br和3-吡啶基在丙酮中进行的SCO温度分别为220、260、215、280和270K。通过几何优化对配体进行了密度泛函理论的分子轨道(MO)计算。吡啶氮原子[ρ(N py)]上的原子电荷是从自然轨道种群分析中提取的。在T 1/2与ρ(N py)图中出现正相关,R 2 = 0.734,这与使用Hammett取代基常数(σp和σp +)的分析一致。这一发现与提出的机理非常吻合:富电子密度提升了t 2g通过dπ–pπ相互作用产生的能级,导致了2 g –e g的窄能隙,并倾向于高自旋态和低T 1/2。MO方法已成功应用于涉及4个取代的2,6-双(吡唑-1-基)吡啶的SCO活性铁(II)化合物。在T 1/2对ρ(N py)图上出现了明显的正相关。相关系数的比较表明,ρ(N py)是比σp或σp +更可靠的参数,可以预测T 1/2的偏移。此外,该方法可以通过应用到具有3-氮烯基-4-对甲苯基-5-苯基-1,2,4-三唑配体系列的另一个已知的SCO家族中来更一般化,其中氮嗪基代表2-氮杂芳族环。线性相关性良好的在发现Ť 1/2与ρ(N甲)情节(N甲处于azaaromatic环结扎氮原子)。最后,我们将说明仅通过考虑电子扰动才能使本治疗方法能够预测SCO平衡位置的原因。
更新日期:2018-06-21
中文翻译:

从DFT计算可预测具有4个取代的Pybox和相关杂芳族配体的铁(II)配合物的自旋交叉温度
自旋交叉(SCO)是在低自旋状态和高自旋状态之间通过外部刺激(例如热)的可逆转变。研究了一系列[Fe II(X-pybox)2 ](ClO 4)2的SCO行为和转变温度(T 1/2),建立了配体场工程学方法,其中X-pybox代表2在吡啶环的4-位被X取代的,6-双(恶唑啉-2-基)吡啶。我们利用X = MeO,Me,3-噻吩基,Ph,H,MeS,2-噻吩基,N 3,Cl,Br,3-吡啶基和4-吡啶基。X = Me,2-噻吩基,N 3的五种新衍生物的溶液磁化法,Br和3-吡啶基在丙酮中进行的SCO温度分别为220、260、215、280和270K。通过几何优化对配体进行了密度泛函理论的分子轨道(MO)计算。吡啶氮原子[ρ(N py)]上的原子电荷是从自然轨道种群分析中提取的。在T 1/2与ρ(N py)图中出现正相关,R 2 = 0.734,这与使用Hammett取代基常数(σp和σp +)的分析一致。这一发现与提出的机理非常吻合:富电子密度提升了t 2g通过dπ–pπ相互作用产生的能级,导致了2 g –e g的窄能隙,并倾向于高自旋态和低T 1/2。MO方法已成功应用于涉及4个取代的2,6-双(吡唑-1-基)吡啶的SCO活性铁(II)化合物。在T 1/2对ρ(N py)图上出现了明显的正相关。相关系数的比较表明,ρ(N py)是比σp或σp +更可靠的参数,可以预测T 1/2的偏移。此外,该方法可以通过应用到具有3-氮烯基-4-对甲苯基-5-苯基-1,2,4-三唑配体系列的另一个已知的SCO家族中来更一般化,其中氮嗪基代表2-氮杂芳族环。线性相关性良好的在发现Ť 1/2与ρ(N甲)情节(N甲处于azaaromatic环结扎氮原子)。最后,我们将说明仅通过考虑电子扰动才能使本治疗方法能够预测SCO平衡位置的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号